
This segment establishes the clinical context for BRAF V600E–mutant metastatic NSCLC, a relatively rare but clinically important subset of lung cancer.

This segment establishes the clinical context for BRAF V600E–mutant metastatic NSCLC, a relatively rare but clinically important subset of lung cancer.
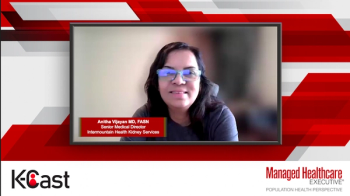
Expert discusses how combining lifestyle and pharmacologic interventions effectively lowers cardiovascular risks and slows CKD progression.

Detailed discussion of Fitusiran, Marstacimab, and Concizumab, their mechanisms of action, differentiation from factor replacement, and highlights from key clinical trials (BASIS, ATLAS, Explorer 8).

Elacestrant significantly improved progression-free survival and maintained a manageable safety profile, especially benefiting patients with ESR1-mutated tumors.

Mark Nestor reveals groundbreaking insights on botulinum toxin's evolving science, enhancing its efficacy and expanding its medical applications in dermatology.

Discover how botulinum toxin injections in the glabella affect brain function, enhancing mood by altering emotional feedback from frowning.

Expert discusses how applying and adhering to established CKD screening guidelines ensures consistent, early detection and better outcomes across care settings.

Expert discusses how, despite its importance, albuminuria screening remains underutilized, and improving adherence to dual testing can transform early CKD detection.

Exploration of the emergence of nonfactor treatment classes, explaining how these agents function independently of missing clotting factors and their relevance to patient convenience, dosing, and efficacy.

Among chemotherapy-naive patients, elacestrant extended median progression-free survival compared with standard therapy, particularly in ESR1-mutated cases.

The EMERALD study carries significant implications across multiple stakeholders in ER+/HER2- metastatic breast cancer.

Expert discusses how understanding and promoting awareness of CKD stages enables earlier intervention and better management of disease progression and cardiovascular risk.

A historical perspective on traditional factor replacement therapies, tracing their development, successes, and ongoing limitations such as short half-life, infusion requirements, and inhibitor formation.

Expert discusses how the high prevalence of undiagnosed CKD creates a significant clinical and financial burden that can be alleviated through earlier detection and intervention.

Milliman’s Jennifer Cruz says plans are losing rebates because of steep price discounts negotiated under the Inflation Reduction Act. but that they need to be sure that renegotiation of rebate guarantees doesn’t get too broad.

Pharmacy benefit managers are approaching health plans with value-based contracts that consider lower costs on the medical benefit of the insurance, says Jennifer Cruz of Milliman.

AI and virtual care revolutionize dermatology, enhancing melanoma detection, expanding patient access and improving efficiency in clinical practices.

Efficacy analyses from the EMERALD trial demonstrated that the oral SERD elacestrant improved progression-free survival, particularly in patients harboring ESR1 mutations, highlighting its potential to overcome endocrine resistance.

Stratification in clinical trials, such as by ESR1 mutation status, presence of visceral metastases, and prior fulvestrant exposure, mirrors key considerations in real-world management of ER+/HER2- metastatic breast cancer.

Nearly all women experience a decrease in libido as they go through perimenopause and menopause, but it remains unaddressed

Fulton County leaders tackle healthcare disparities by forming partnerships and enhancing access, aiming for equitable health solutions in Georgia. Pamela Roshell, Ph.D., and LaTrina Foster spoke with Managed Healthcare Executive in this second part of a two-part video interview series.
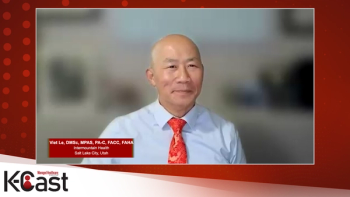
An expert discusses how the CLEAR Outcomes trial data demonstrated that bempedoic acid effectively reduces cardiovascular events by 13% in nearly 14,000 statin-intolerant patients over 4.9 years with excellent tolerability, while long-term data from PCSK9 inhibitor studies such as the FOURIER trial show sustained safety and efficacy over five to eight years with the principle that “lower for longer is better,” as patients who started treatment earlier maintained cardiovascular protection advantages that late-starters could never catch up to despite achieving similar LDL reductions.

Fulton County, Georgia addresses health disparities through innovative partnerships, expanding access to care and a holistic approach to behavioral health services, according to county experts LaTrina Foster and Pamela Roshell, Ph.D., in this first part of a two-part video series.

Although people are living longer with HIV, patients must still grapple with survivors’ remorse and the challenges of growing older within an ageist society.

Milliman's Jennifer Cruz, Pharm.D., said health plans should be able to track rebates to their book of business and have strong audit rights.

The EMERALD trial enrolled patients with ER+/HER2- metastatic breast cancer who had progressed on prior endocrine therapy, including mandatory exposure to a CDK4/6 inhibitor, reflecting a modern and clinically relevant population.

The EMERALD trial, which evaluated an oral selective estrogen receptor degrader (SERD) versus standard-of-care endocrine therapies, features several notable design strengths.
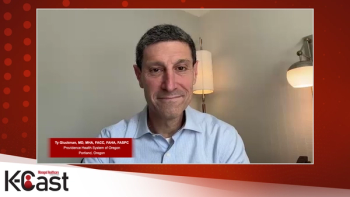
An expert discusses the critical need for a holistic, multidisciplinary approach to prevent and manage interconnected cardiovascular, kidney and metabolic diseases, emphasizing early identification, coordinated care teams and integrated healthcare systems to improve patient outcomes.

An overview of the biological and clinical distinctions between hemophilia A and B, including factor deficiencies, prevalence, diagnostic nuances, and implications for treatment strategy.

An expert discusses how the “lower for longer is better” principle drives current guideline updates, with the National Lipid Association and American Diabetes Association now recommending more aggressive LDL cholesterol targets (less than 70 mg/dL for patients with diabetes) and earlier initiation of combination therapies. The recent 2025 ESC guidelines establish even lower targets, including less than 40 mg/dL for extreme-risk patients and recommend bempedoic acid as monotherapy for statin-intolerant patients or in combination therapy, with oral medications preferred before injectables.