Spinal Muscular Atrophy
Latest News
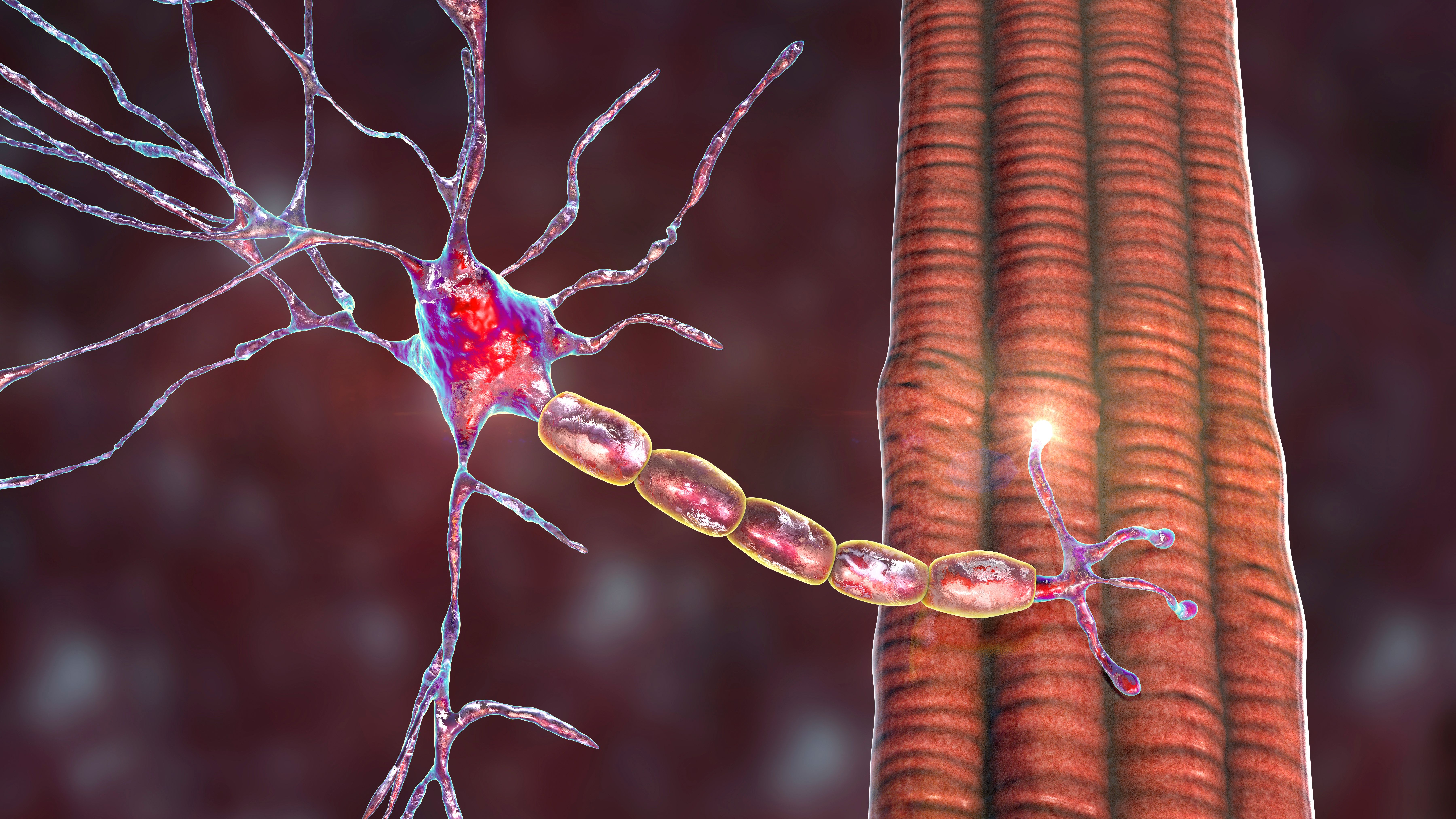
Video Series
Latest Videos
CME Content
More News

Roche’s Evrysdi (risdiplam) is the market leader among the drugs for spinal muscular atrophy (SMA). Biogen’s Spinraza (nusinersen) has struggled, but company officials express confidence about future sales.

In a response to a survey, caregivers of people with spinal muscular atrophy identified the risk of severe adverse events and the need for permanent ventilation as the most important factors in treatment decisions. Access to treatment, including cost and availability, ranked third.

Some prior research had shown that gene therapy would not lower the amount of time children spend on ventilator support.

Two years after treatment, both girls show normal motor function and no signs of spinal muscular atrophy symptoms.

Apitegromab works by targeting a protein called myostatin that limits muscle growth and is designed to be used alongside existing spinal muscular atrophy (SMA) treatments.

The higher dose led to a more rapid decline in an important marker of neurodegeneration, according to a Biogen news release announcing the results.

Study of 29 children treated with Zolgensma (onasemnogene abeparvovec) shows mixed results.
U.S. providers surveyed said that getting insurance approval is time-consuming and can delay treatment for newborns with spinal muscular atrophy.

Data from the DEVOTE study suggest that 50-milligram doses are safe and effective.


Apitegromab, an investigational drug for spinal muscular atrophy, demonstrated sustained patient benefits and maintained safety over a three-year period in a phase II study, with 88% of participants maintaining or improving motor milestones.

The system, called ThecaFlex DRx, allows subcutaneous administration of Spinraza (nusinersen) directly into the cerebrospinal fluid, and aims to replace the current administrative route through lumbar punctures.


SMA, a rare genetic neurodegenerative disorder affecting approximately 1 in 10,000 newborns, is typically fatal for untreated children by age two. However, the approval of disease-modifying medications have transformed the treatment landscape.

Risdiplam is an oral small molecule treatment that impacts both the central nervous system and peripheral tissues. Patients receive the medicine daily in liquid form either by feeding tube or by mouth. The drug was first approved by the FDA in 2020 for patients as young as two-months old.
According to a study published in JAMA Pediatrics, screening newborns for spinal muscular atrophy (SMA) and starting treatment immediately can significantly improve health outcomes compared to infants who are diagnosed and begin treatment months later.

Early treatment helps improve the efficacy of treatment of spinal muscular atrophy, according to a comprehensive review article published earlier this month.
In this diagnostic proof-of-concept trial, an emerging imaging approach that uses a handheld laser demonstrated the ability to visualize muscle degeneration in children with SMA.

A new study suggests clinicians might be able to use metabolic “fingerprints” to better understand the likely severity of individual cases.
Investigators in Japan have developed a new, noninvasive method for screening for spinal muscular atrophy using saliva analyzed with conventional PCR as an alternative to blood-based genetic screening.

This European study enrolled patients with more severe cases of spinal muscular atrophy than a similar study conducted in the U.S.
A new method for delivering this treatment drug for spinal muscular atrophy offers an option for patients with advanced cases of the disease. But investigators found that the new method may also carry risks of mechanical failure and infection.
Small biotech companies such as Scholar Rock and Cytokinetics have treatments in late-stage trials for spinal muscular atrophy. Novartis and Biogen also have products in the pipeline.

The FDA has approved three targeted therapies for spinal muscular atrophy. They have hefty price tags so cost and affordability are live topics.
The phase 2 TOPAZ trial demonstrated treatment is safe and improves motor function in patients with SMA.