
Some vials in two lots of the antibiotic cefazolin are labeled as being penicillin G potassium, which if used could lead to suboptimal outcomes, adverse events, drug interactions and delayed recovery.

Some vials in two lots of the antibiotic cefazolin are labeled as being penicillin G potassium, which if used could lead to suboptimal outcomes, adverse events, drug interactions and delayed recovery.

The FDA has seized several hundred units of counterfeit Ozempic 1 mg
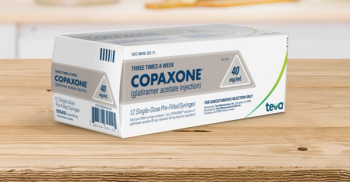
Regulatory officials have identified 82 cases worldwide from the FDA Adverse Event Reporting System of anaphylaxis associated with the use of Coxapone and the generic Glatopa that have resulted in hospitalization or death.

Regulators say, however, the benefits of vaccination with Abrysvo and Arexvy in preventing respiratory syncytial virus continue to outweigh their risks.
The boxed warning follows a safety warning the FDA issued in September about the risk of liver injury from the use of Veozah to treat hot flashes.
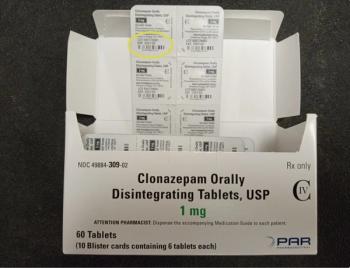
A total of 17 lots are now part of the recall of Clonazepam because some cartons have the wrong strength on the label.

Gavreto used to treat metastatic RET fusion-positive non-small cell lung cancer and RET fusion-positive thyroid cancer.

Clinical data suggests an imbalance in vaso-occlusive crises and fatal events that require further assessment, and the company said it will provide updates in the future.
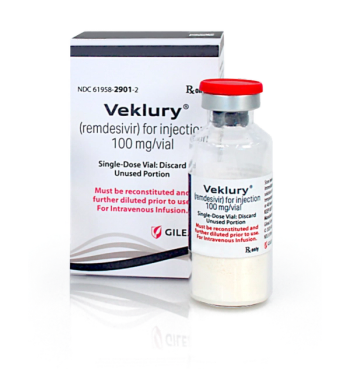
Veklury is indicated to treat adults and children who are hospitalized with COVID-19.
The FDA has identified a probable case of serious drug induced liver injury that occurred in a woman in the United States who had received Veozah. The agency is requiring additional liver blood testing after starting therapy.

The recalled batches have been found to have potassium chloride that does not dissolve, which can cause high potassium levels that can lead to hypertension, heart failure, or renal dysfunction.

Dr. Reddy’s is recalling five lots of Javygtor and one lot of generic sapropterin dihydrochloride because discoloration could lead to decreased potency. Sapropterin is used to treat an inherited metabolic disorder.

An additional seven lots of Treprostinil are affected on top of last month’s recall of one lot.
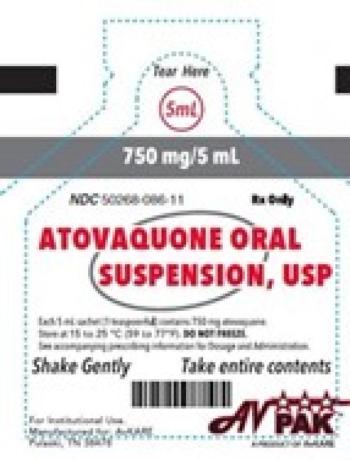
Bacillus cereus contamination in Atovaquone Oral Suspension, which treats AIDS-related pneumonia, was found during stability testing at a third-party lab.

The recommended maximum daily dose of Vancomycin is up to 2 grams a day. The overfilled vials could result in patients receiving up to 4 grams a day.

One lot of treprostinil injection is being recalled. Tresprostinil is a prostacyclin vasodilator used to treat patients with pulmonary arterial hypertension.
The FDA orders new warnings for CAR-T cell therapies due to reports of T-cell malignancies, including fatal cases, applying to BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies.

The antihistamine drug carbinoxamine was found in one bottle Zenzedi, which is used to treat narcolepsy and ADHD.

Regulators are warning about the increased risk of very low blood calcium levels in patients with advanced chronic kidney disease.

Regulators will continue to evaluate a possible link by reviewing meta-analysis of clinical trials across all GLP-1 products and analyzing postmarketing data in the Sentinel System.
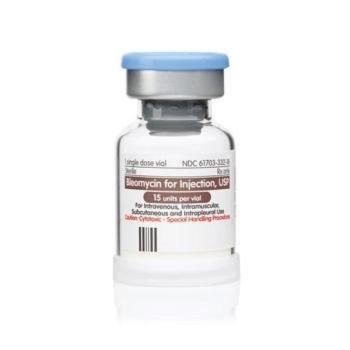
The presence of glass can lead to serious adverse events, including inflammation of a vein and blockage of blood vessels or life-threatening blood clots.

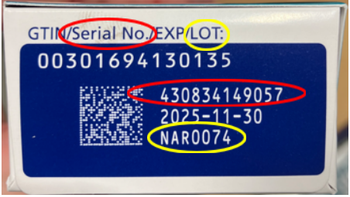
The FDA has seized thousands of units of counterfeit Ozempic 1 mg.
Cipla is recalling one lot of vigabatrin, which is used to treat seizures.

Shortages are growing because of disruption caused natural disasters and ingredient supply issues, as well as increased demand for certain drugs.