
To date, Pfizer has not received reports of adverse events related to this recall, but is making the move as a precaution.

To date, Pfizer has not received reports of adverse events related to this recall, but is making the move as a precaution.

The affected lots of this rare disease therapy have been contaminated with yeast, mold, and bacteria, which could lead to life-threatening infections.

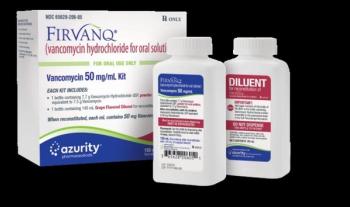
The lot contains kits with the incorrect solution for diluting vancomycin, which could lead to doses above or below recommended levels.


The FDA’s review of the JAK inhibitor class concluded there is an increased risk of serious heart-related events such as heart attack or stroke, cancer, blood clots, and death with these arthritis and ulcerative colitis medicines.

The product is considered “super potent” with a higher dose that could result in serious cardiac toxicities.
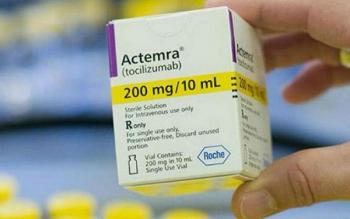
Pharmacists are referring patients elsewhere or are shifting patients who were using the IV form to the subcutaneous injection. The NIH has also recommended using Kevzara as an alternative where there are shortages of Actemra.

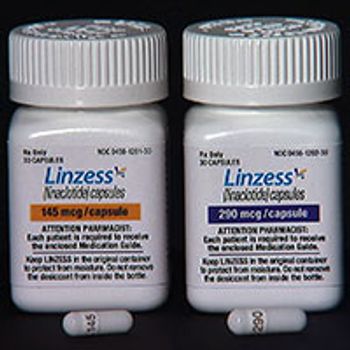
The update indicates there is a serious risk of dehydration and is contraindicated in children.

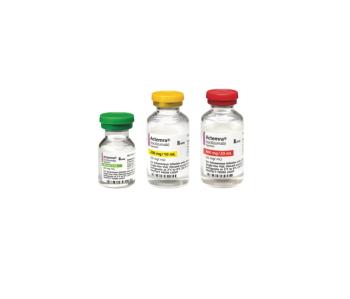
The monoclonal antibody had been given an emergency use authorization to treat COVID-19, but the recent spike in cases is driving a shortage in supply.

A total of 16 lots have been recalled.
The bottles have a counterfeit foil seal or label and contain an incorrect number of tablets.

The therapy for HIV-related pneumonia was exposed to cold weather during shipment.
Some fatal cases of ketoacidosis have occurred, and high-risk patients should be monitored when starting treatment.
The agency has stopped enrollment in all ongoing trials.
Angiotensin receptor blockers may be less likely to cause side effects than ACE inhibitors.

Keytruda gets a full approval in endometrial cancer; while Xeljanz will miss another PDUFDA date.

Most of the suspected cases of myocarditis developed after the second dose of the vaccine and were among younger males.
This is being done in coordination with the FDA to address the shortage created by the recall of Chantix. UPDATE: Pfizer has expanded its recall of Chantix from nine lots to 12 lots.

This research sought to understand the relationship of prior medication exposure, existing health conditions, and COVID-19 outcomes using data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry.
An FDA inspection of compound pharmacy Innoveix Pharmaceuticals indicated these lots may not be sterile.

Jardiance lowered risk of hospitalization from acute kidney injury but increased risk of hospitalized from diabetic ketoacidosis compared with a DPP4 inhibitor in one interim analysis.
While the risk of exposure is low, it presents a severe hazard.