
While heritable human genome editing is banned in the United States, across Europe and much of the world, the technology raises concerns about potential misuse.


While heritable human genome editing is banned in the United States, across Europe and much of the world, the technology raises concerns about potential misuse.
Scientists working at the NIH’s BRAIN Initiative have created new viral vectors that one day could lead to gene therapies for ALS, seizure disorders, Parkinson’s and Alzheimer’s disease, Huntington’s disease and other diseases of the brain.
A personalized gene editing therapy was successfully used to treat an infant with CPS1 deficiency, a disease that causes ammonia buildup in the blood. The technology used creates an opportunity for the development of personalized treatments for other rare diseases.

Healthcare providers are exploring ways to reduce patient travel through a growing trend of expanding sites of administration beyond academic medical centers into community and outpatient settings closer to patients' homes.

Plans expect cell and gene therapies to pose a moderate or major financial challenge in the next two to three years, according to a new survey of pharmacy benefit leaders by PSG.
A phase 1b showed promising results in lowering LDL in patients with heterozygous familial hypercholesterolemia and/or premature coronary artery disease.
Lexeo Therapeutics’ gene therapy provides a healthy version of the frataxin gene, which is critical for energy production in cells. The company plans to begin a pivotal trial next year.

None of the cell and gene therapies that reached the final regulatory submission stage were rejected by the FDA.

Encelto is small, semi-permeable capsule implanted in the eye that contains allogeneic retinal pigment epithelium cells genetically engineered to produce specific therapeutic proteins. It will be available in June 2025.
EsoBiotec’s platform has the potential to eliminate the complex and time-consuming process for manufacturing, helping to make CAR-T cell therapies more accessible.
Early research shows a one-time gene therapy has potential for addressing both the liver and lung disease that patients with alpha-1 antitrypsin deficiency (AATD) face.

Researchers have suggested that uniform evidence standards and reporting requirements could help speed patient access to cell and gene therapies and help ensure consistent safety and efficacy evaluations.

Solid Biosciences’ gene therapy uses a shortened version of the dystrophin protein, which is engineered to mimic the key functions of full-length dystrophin and allows it be delivered more effectively to muscle cells.
A mouse study at Memorial Sloan Kettering Cancer Center has found that adding Nef, a protein from HIV, allows donor CAR T cells to avoid detection by the immune system while still attacking tumors.

The majority are in the very early preclinical stage of development, but applications for regulatory approval have been submitted for 11 gene therapies worldwide, and 35 are in phase 3 trials, according to report delineating the gene therapy development landscape.

Here are a few gene and cell therapy clinical trials with expected data readouts in 2025 that are worth keeping an eye on.
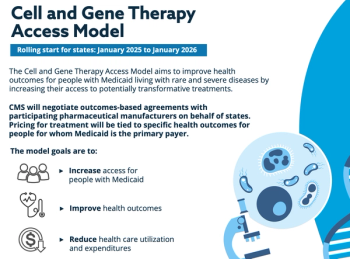
President Trump signed an executive order on the day of his inauguration that created doubts about the future of a program that helps state Medicaid programs pay for expensive cell and gene therapy.
Cardiomyopathies currently have no cures, but many of these disorders are prime candidates for gene therapies.
Gene therapies are revolutionizing treatment for hemophilia A and sickle cell disease; however, they are saddled with hefty price tags and limited patient populations that are impacting uptake.
Research at the annual meeting of the American Society of Hematology evaluated patient and caregiver perspectives on gene therapies for sickle cell disease, which offer great potential but have had slow uptake.

Gene therapies have a lot of potential to improve health outcomes for patients with life-threatening diseases but face a number of barriers that restrict access.

Kebilidi is a one-time gene therapy administered directly to the brain in patients with AADC deficiency, rare genetic disorder.

In a phase 2 trial, a gene therapy reduced angioedema attacks, which could significantly change the quality of life for patients with hereditary angioedema.

In-depth interviews and an online survey found support for gene therapy as a treatment for inherited blood disorders, but also several obstacles that must be addressed.

Up to half of patients showed significant improvement in study results presented at the American Academy of Ophthalmology (AAO) annual meeting