Biologics
Latest News
Latest Videos

CME Content
More News

Studies measuring patient preference stacked up in subcutaneous administration’s favor, according to a recently published review. The difference between subcutaneous and intravenous administration were less clear cut among the studies measuring health-related quality of life.
Pharmaceutical companies are making investments in research of therapies that target three different antigen-binding sites. Trispecific antibody research is still in its earliest phases and is focused on applications in cancer, inflammatory conditions and infectious diseases.

They work by inhibiting inflammatory cytokines. But in a small percentage of patients antiinflammatory biologics may stir up “paradoxical psoriasis.”

Merck and Johnson & Johnson have both acquired bispecific antibodies with applications in autoimmune and inflammatory conditions.
Driving this growth will be the approvals of novel biologics, such as gene therapy, RNA-based therapeutics, and antibody-drug conjugates.
Nine of the 10 top specialty drugs by expenditure are biologics, according to the Pharmaceutical Strategies Group report on specialty drug spending.
The number of biologics approved as treatments for psoriasis has proliferated but the FDA has approved just four for use in children. Six biologics for pediatric psoriasis are in various stages of development.
The FDA has required all approved CAR T therapies to have a boxed warning about the risk of secondary malignancies after treatment.

Pharmacy benefit managers (PBMs) are slowly coming around and putting biosimilars on their formularies. IQVIA estimates that the dearth of a Humira (adalimumab) biosimilars last year cost patients and employers cumulatively $6 billion.

An updated draft guidance says that promotional materials that suggest a biosimilar or reference product is superior “are likely to be false or misleading.” The agency has also warned against making comparisons between biosimilars and those with the interchangeable designation.

A recent IQVIA report found that uptake of biosimilars on the market for at least three years varies from 8% for insulin lispro to 82% for bevacizumab.
The authors of an article about relatively low biosimilar use by people with inflammatory bowel disease have suggestions for how to mitigage the nocebo effect.

Research published in JAMA Health Forum shows that biosimilar savings have not trickled down to out-of-pocket savings for patients.
Next year is shaping up to be a big one for Stelara (ustekinumab) biosimilars. There are also eight biosimilars to Eylea (aflibercept) in the wings, five of which may be approved by the FDA this year.

Ten biosimilars to Humira are now on the market, but AbbVie’s Skyrizi and Rinvoq are soaking up revenues as Humira’s sales fall off.

Research underscores concerns that cost-sharing policies may contribute healthcare disparities.
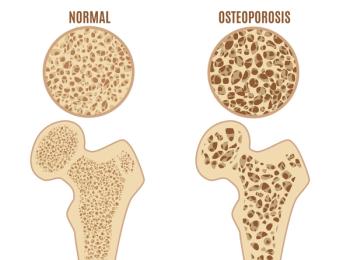
But Amgen and Sandoz are in a patent fight over the launching of Sandoz's biosimilars to the bone-building drugs.

Zymfentra, with a price of $6,181.08 for two shots over four weeks, is approved as maintenance therapy for adults with ulcerative colitis and Crohn’s disease.
The market for Humira biosimilars may be shrinking as AbbVie works to boost prescriptions for its next-generation anti-inflammatories, Skyrizi (risankizumab) and Rinvoq (upadacitinib).

The submission is for the same indications, including Xolair’s recently approved indication IgE-mediated food allergy.
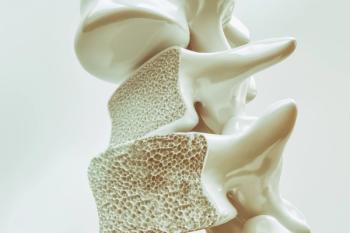
Both Wyost and Jubbonti are interchangeable for the reference products and are approved for all of the same indications in osteoporosis and bone cancer.

At the moment, Simlandi (adalimumab-ryvk) has the high-concentration, citrate-free, interchangeable Humira (adalimumab) market to itself.

The large pharmacy benefit managers have switched up their coverage of biosimilars, especially the biosimilars to Humira (adalimumab).
Acceptance is greater in oncology than in other specialties.

How an open marketplace impacts future competition, pricing transparency, and formulary management.