
Identifying what is truly treatment resistant depression is difficult given all the factors that may influence how a person responds to treatment.

Identifying what is truly treatment resistant depression is difficult given all the factors that may influence how a person responds to treatment.

In contrast to electroconvulsive therapy, patients are not sedated during the treatments. Studies of a protocol called accelerated intermittent theta-burst stimulation have produced positive results.

Various treatment guidelines for depression recommend exercise, but they tend to be short on details

AI (artificial intelligence) can improve the accuracy of disease prediction by crunching volumes of existing clinical data on patients.

One area of interest is whether ketogenic diets, which are high in fat and low in carbohydrates, might be helpful to people with treatment-resistant depression.

Some study results tempered enthusiasm for one digital therapeutic but others are showing promising if early results.

Studies conducted in Taiwan and the United Kingdom have added to. the evidence that inherited genes play a prominent role in the development of treatment-resistant depression.

Spravato is a derivative of the anesthetic ketamine.

The FDA rejected an application to approve MDMA, also known as ecstasy, as a treatment for PTSD. Next up among the psychedelics vying for FDA approval: psilocybin for treatment resistant depression.
Biosimilars generally enter the market priced at roughly 50% of the amount of their reference drugs.
The market for biosimilars seems to be improving, partly because of provisions in the Bipartisan Budget Act of 2018 and the Inflation Reduction Act of 2022.

Pharmacy benefit managers (PBMs) are slowly coming around and putting biosimilars on their formularies. IQVIA estimates that the dearth of a Humira (adalimumab) biosimilars last year cost patients and employers cumulatively $6 billion.

An updated draft guidance says that promotional materials that suggest a biosimilar or reference product is superior “are likely to be false or misleading.” The agency has also warned against making comparisons between biosimilars and those with the interchangeable designation.
The authors of an article about relatively low biosimilar use by people with inflammatory bowel disease have suggestions for how to mitigage the nocebo effect.

Research published in JAMA Health Forum shows that biosimilar savings have not trickled down to out-of-pocket savings for patients.
Next year is shaping up to be a big one for Stelara (ustekinumab) biosimilars. There are also eight biosimilars to Eylea (aflibercept) in the wings, five of which may be approved by the FDA this year.

Ten biosimilars to Humira are now on the market, but AbbVie’s Skyrizi and Rinvoq are soaking up revenues as Humira’s sales fall off.
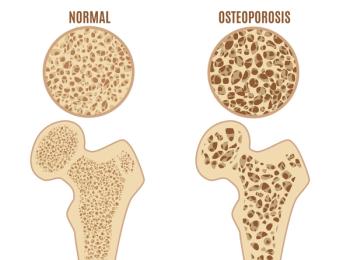
But Amgen and Sandoz are in a patent fight over the launching of Sandoz's biosimilars to the bone-building drugs.
The market for Humira biosimilars may be shrinking as AbbVie works to boost prescriptions for its next-generation anti-inflammatories, Skyrizi (risankizumab) and Rinvoq (upadacitinib).

There is no vaccine against the hepatitis C virus. A subscription-like payment model may help overcome some of the cost issues posed by direct-acting antivirals.
Acceptance is greater in oncology than in other specialties.
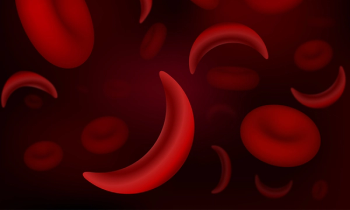
Two possibly curative gene therapies for the disease, which disproportionately affects Black people, are under consideration by the FDA.
The National Bleeding Disorders Foundation (NBDF) has joined a petition demanding that Payer Matrix stop representing itself as a patient advocacy organization.
Despite positive safety and quality of life results for FEIBA, the emergency of Hemlibra created difficulties for the FEIBA study and resulted in it being halted.

Joe Pugliese, president and CEO of the patient advocacy group Hemophilia Alliance, discusses recent announcements by manufacturers of two ultra-expensive gene therapies for hemophilia that they will offer payers partial or full reimbursement if patients fail on their therapies.

BioMarin Pharmaceutical and CSL Behring are offering valu\ed-based contracts for their hemophilia gene therapies.

The British cost-effectiveness agency said the evidence from a pivotal clinical trial wasn't strong enough to clarify Hemgenix's effectiveness relative to other therapies or satisfy concerns about its long-term durability.
University of Pittsburgh’s Margaret Ragni, M.D., M.P.H., discussed fitusiran, the investigational small interfering RNA (siRNA) therapeutic, with Managed Healthcare Executive and also some of the possible limitations of gene therapy for hemophilia.

A yearlong study of the newly approved half-life extender showed no evidence of factor VIII inhibitors.

Emissions from healthcare facilities contribute to climate change, and climate change, in turn, is presenting new challenges for healthcare.
Published: July 18th 2022 | Updated:
Published: March 8th 2023 | Updated:

Published: November 18th 2024 | Updated:
Published: November 13th 2022 | Updated:
Published: March 21st 2024 | Updated:

Published: October 22nd 2024 | Updated: