
Shekhar Khera, senior vice president, product management at RxBenefits, discusses the role of artificial intelligence in healthcare and how his company is using it to improve efficiency, outcomes and the member experience.

Shekhar Khera, senior vice president, product management at RxBenefits, discusses the role of artificial intelligence in healthcare and how his company is using it to improve efficiency, outcomes and the member experience.


Alexa B. D’Angelo, Ph.D., M.P.H. and Jeremiah Johnson share their predictions for HIV challenges in 2026.

Experts predict shifts in U.S. healthcare for this year, focusing on affordability, consumer demands, mental health equity and the essential role of doulas.

The oral versions may set records in 2026, and glucagon-like peptide 1 (GLP-1) drugs will pick up even more momentum as medications for cardiovascular risk reduction as well as obesity, predict experts.

Transthyretin amyloid cardiomyopathy causes heart failure through protein buildup in heart muscle. Three FDA-approved disease-specific therapies are available, but high costs limit access.

Write-ups of presentations on the 340B Drug Pricing Program were among the most viewed conference-related articles on the MHE website in 2025.

The most-viewed articles from our print publication included our November 2025 cover story on AI and our April 2025 cover story on Medicaid cuts.

Lucille Accetta, RPh, M.P.H., MBA, of CVS Health, foresees more progress in the push for simplification. A.J. Loiacono of Judi Health and Capital Rx, predicts unified management of healthcare financing and benefits on one "healthcare operating system."

Predictions from Tracy Baroni Allmon, BS Pharm, Eva Borden, Sharon Faust, Mike Kolodij, and James Margiotta

The top news to come out of skin cancer coverage this year for MHE focused on blood or marrow transplant recipients experiencing increased skin cancer risk, highlighting the need for vigilant monitoring and innovative treatment strategies.

Predictions from Marci Chodroff, M.D., Jeffrey Casberg, RPh, M.S., Sarah Emond, M.P.P., Adam Kautzner, Pharm.D., David Fields, Nate Karnitz, MBA, and David Blair

New studies from the year revealed connections between medications, environmental factors and innovative treatments for skin conditions including vitiligo and atopic dermatitis.

GLP-1 obesity drugs saw shifting coverage and access in 2025, with low long-term adherence, payer cost concerns, new generics and key regulatory and formulary moves shaping use.

In 2025, respiratory news highlighted advances in lung imaging, expanded support for pulmonary rehabilitation and promising new and repurposed therapies for pulmonary fibrosis.

This year in type 1 diabetes, researchers advanced immune-reprogramming nanoparticle therapies and hypoimmune stem cell transplants, launched pivotal islet cell therapy trials, updated screening and treatment guidelines, and warned that cuts to research funding could slow future innovation.

Breakthrough treatments, shifting care models and persistent system challenges defined cancer care this year, from evidence that CAR T-cell therapy can lower costs and treatment burden in mantle cell lymphoma to promising off-the-shelf cancer vaccines.

This year, we explored how innovations in women's health are bridging care gaps, addressing biases and enhancing access to vital treatments and screenings.

Tylenol and autism, the Inflation Reduction Act, the One Big Beautiful Bill, Most Favored Nation pricing and direct drug sales to consumers were some of the biggest news items we covered in 2025.

Medicare price negotiations, higher costs driven by cancer and GLP-1 drugs and Medicare’s pilot program for obesity drugs are some of the biggest trends this year that will continue to impact managed care in 2026.

Transparency, prior authorization, PBM reform and PBM offered genomics testing are some the trends Formulary Watched tracked in 2025.
Pfizer’s sigvotatug vedotin combined with Keytruda is being studied in patients with non-small cell lung cancer. Researchers suggest sigvotatug vedotin’s ability to induce immunogenic cell death may enhance antitumor activity.

Researchers wanted to provide insight into the relative clinical value of Apretude and lenacapavir compared with no pre-exposure prophylaxis (PrEP), and they presented the data at the International AIDS Society meeting.
A separate analysis of the real-world trial found that those who switched from daily antiretroviral treatment to Cabenuva had fewer concerns about treatment.


In an interview with Managed Healthcare Executive, Christian T. Ruff, M.D., M.P.H., director of general cardiology at Brigham and Women's Hospital and associate professor at Harvard Medical School, addressed the uncertainty of the bleeding risks of abelacimab compared to rivaroxaban and highlighted abelacimab’s safety profile.
Patients with atopic dermatitis who had not received biologics prior to treatment with Opzelura were able to avoid biologics during the 12 months after treatment with the topical nonsteroidal, according to study findings presented at the 2025 American Academy of Dermatology annual meeting.

The reduction in itch was the greatest contributor to quality-of-life improvement for adolescents and adults with atopic dermatitis, according to a new study presented at the American Academy of Dermatology annual meeting.

The year's top conference coverage item shares the effects of "Ozempic Bod" and "Ozempic Face," as presented at the annual American Academy of Ophthalmology meeting in Chicago.

Check out this list of the top five biologics articles posted on Managed Healthcare Executive this year!

Published: June 7th 2021 | Updated:

Published: March 6th 2024 | Updated:

Published: November 14th 2021 | Updated:
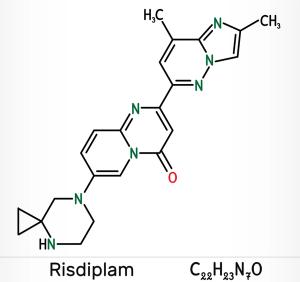
Published: August 10th 2020 | Updated:

Published: September 13th 2020 | Updated:
Published: August 7th 2020 | Updated: