
François de Brantes, M.S., MBA is a senior partner at HVC Incentives Advisory Group LLC and a member of Managed Healthcare Executive editorial advisory board. He predicts "price distortions will be revealed" in 2024.

François de Brantes, M.S., MBA is a senior partner at HVC Incentives Advisory Group LLC and a member of Managed Healthcare Executive editorial advisory board. He predicts "price distortions will be revealed" in 2024.

Fran Gregory, Pharm.D., MBA is the Vice President of Emerging Therapies at Cardinal Health. She thinks 2024 will be an exciting year for cell and gene therapy.

Melynda Barnes, M.D. is the Chief Medical Officer at Ro. She believes "more people will gain access to GLP-1 inhibitors" in 2024.

Michael Meucci is the CEO of Arcadia. He thinks that EHRs will get an AI makeover.


Maulik P. Purohit, M.D., M.P.H., is the Chief Health Information Officer at the Lehigh Valley Health Network. He believes that "AI will enhance the patient experience" in 2024.

Joerg Schwarz, M.S., is the Senior Director of Healthcare Interoperability at Infor. He believes providers will harness the power of generative AI in 2024.

Brianna Zink, M.S.N, is the Senior Director of Health Care Product Strategy at Infor. She says staff ratios will become a burden in 2024.

This year's top multimedia content includes a video interview with Jennifer Roggenbuck, MS, LGC, of Ohio State University, who addresses the patient journey of ALS.

Laura Kline, MBA, CPCU is the Senior Vice President of Business Development at The Doctors Company. She predicts "APCs will be critical to access care."

Casberg is Vice President of Clinical Pharmacy at IPD Analytics LLC and a member of the Managed Healthcare Executive editorial advisory board. He predicts that "payers will tighten" in 2024.

This year's top HIV articles include one sharing data from a group of researchers from University of Pittsburgh who looked to see if T cell activation is sufficient to drive SIV disease progression.

Kelsay is the President and CEO of the Business Group on Health. She predicts that in 2024, employers will "sound the drug price alarm."

Robert E. White Jr. is the President of The Doctors Company. He believes "social inflation will affect malpractice insurers."

This year's top oncology news included a study that suggested low-fat meals could affect blood concentration levels, and high blood concentration has been associated with a better response to the drug, Alecensa.

Weight-loss medications were very much in the news this year, and the pages and website of Managed Healthcare Executive were no exception. Articles on off-label use of the GLP-1s such as Ozempic (semaglutide) and the FDA approval of Zepbound (tirzepatide) garnered the most page views.

Articles about Humira biosimilars, over-the-counter hearing aids and specialty drugs were among those about healthcare and drug costs that had the most pageviews on the MHE website in 2023.

News about state bans on accumulators, Pfizer’s announcements about vaccines and cardiovascular deaths increasing because of climate change were the most viewed news items on the Managed Healthcare Executive website this year.

Articles about upcoming approvals and Humira biosimilars were among those that garnered the most interest.
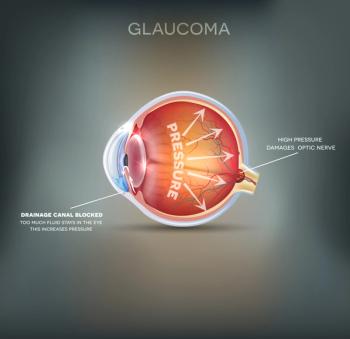
IDose TR will be available in first quarter of 2024 with a wholesale acquisition cost of $13,950 per dose/implant.

Rising healthcare costs impact their own organization’s competitiveness, said those who responded to the Pulse of the Purchaser survey by the National Alliance of Healthcare Purchaser Coalitions.

Mike Tuffin is set to become AHIP's President and CEO from January 8, 2024, succeeding Julie Simon Miller.
Marstacimab is being reviewed to prevent or reduce the frequency of bleeding episodes in people with hemophilia A or B. The FDA has set an action date in the first quarter of 2024.

Joe Kvedar, M.D., the immediate past chair of the association, is chairing the 35-member group that will be focused on eliminating barriers to telehealth and other types of virtual health.

In response to the high demand for healthcare professionals and over 2 million annual job openings — a 40% growth rate surpassing the average for all other occupations—healthcare organizations are seeking technological solutions to address this pressing issue.
If approved, elafibranor would be a second-line treatment for patients with primary biliary cholangitis. The Prescription Drug User Fee Act action date is June 10, 2024.
Acoramidis is an oral small molecule to treat patients with transthyretin amyloid cardiomyopathy.

Pharmacy benefit managers have been under pressure to simplify their complicated, obscure pricing tactics.

The survey highlights a growing willingness among employees to pay for premium lens options and a focus on early diagnosis of eye conditions, aligning with the broader trend of holistic well-being.

Cigna has been looking to sell its Medicare Advantage business, perhaps as a way to fend off antitrust objection to the deal.