
2024's top podcast episodes includes a conversation with Paul Fronstin, director of health benefits research at EBRI, who shed light on the evolving landscape of health benefits.

2024's top podcast episodes includes a conversation with Paul Fronstin, director of health benefits research at EBRI, who shed light on the evolving landscape of health benefits.

Take a look at this year’s top articles — one showcasing enhanced weight loss through Semaglutide use in post-menopausal women on hormonal therapy.

This year’s top dermatology posts on Managed Healthcare Executive include stories about Gen Z sunscreen trends and increasing incidences of ringworm and jock itch.

Here are the top performing articles about obesity published this year on Managed Healthcare Executive.

One of this year's top articles on cost and coverage highlights that the average American can afford a maximum of $97 in out-of-pocket healthcare expenses.

Of the top news articles, Vertex Pharmaceuticals' suzetrigine garnered much attention as it's a promising new class of pain medicine that might offer hope for those struggling with acute pain issues who want to avoid opioids and NSAIDs.

Check out this list of the top five articles posted on Formulary Watch this year!

Take a look at the best performing print articles of 2024. This year's top article spotlights the surge of mRNA vaccines being developed for other diseases outside of COVID-19.

CVS Health recently appointed Len Shankman as president of Pharmacy and Consumer Wellness and Lucille Accetta as chief pharmacy officer.

Effective July 1, 2025, this agreement ensures that nearly 400,000 patients covered by IBX each year will keep receiving quality care from Penn Medicine providers. The partnership focuses on improving health outcomes, patient experiences, and controlling costs, aiming to set new standards for innovative care in healthcare, according to a release.
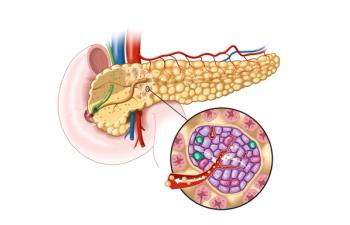
Early research shows the CD40L antibody tegoprubart is able to reduce insulin dependence in patients with type 1 diabetes after islet transplants.
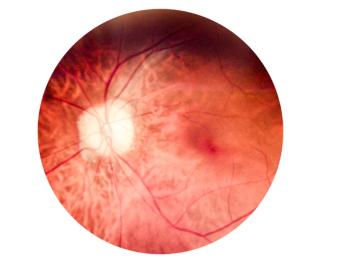
Medicare patients are more likely to receive aflibercept 2 mg over other anti-VEGF agents compared with commercially insured patients.

After expansion of Medicaid, there was a significant decrease in the low-income population with no healthcare coverage.
Patients with HIV who receive Cabenuva injections are more closely followed by physicians to assess the risk of cardiovascular, liver and kidney disease.

The survey also found that flexible and virtual appointments, as well as transportation support, were helpful while on injection long-acting PrEP.

The higher dose led to a more rapid decline in an important marker of neurodegeneration, according to a Biogen news release announcing the results.
Jill Zouzoulas, M.D., of Tryon Medical Partners in Charlotte, North Carolina, and Jennifer Seminerio, M.D., of University of Southern Florida Health in Tampa, Florida, discussed the biologics and the pros and cons of the intravenous and subcutaneous delivery methods in Managed Healthcare Executive’s K-Cast video series.
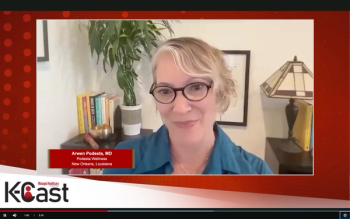
Arwen Podesta, M.D., discussed prescription digital therapeutics for people with mental health and substance abuse conditions in a Managed Healthcare Executive K-Cast video series. Podesta is a New Orleans-based board-certified adult psychiatrist with subspecializations in addiction medicine, forensic psychiatry and integrative medicine.
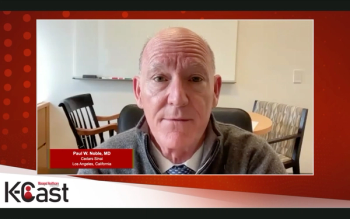
Paul W. Noble, M.D., of Cedars-Sinai in Los Angeles, and Paul Frohna, M.D., Ph.D., of Endeavor BioMedicines, discussed the symptoms, epidemiology diagnosis and treatment of idiopathic pulmonary fibrosis (IPF) in a Managed Healthcare Executive K-Cast video series.

Sean M. Oser, M.D., M.P.H., an associate professor in the Department of Family Medicine at the University of Colorado School of Medicine, discussed diabetes management and quality measures in a Managed Healthcare Executive K-Cast video series.

Data from the DEVOTE study suggest that 50-milligram doses are safe and effective.

Researchers surveyed 201 men who have sex with men and transgender men from 17 clinics who began long-acting cabotegravir to prevent infection with HIV.

Lotilaner ophthalmic solution 0.25% drops are administered as twice daily for six weeks. Other ways of treating Demodex blepharitis manage symptoms, not the root cause, and are of indeterminate length.

Here’s what you missed this week on Managed Healthcare Executive.

A separate trial is ongoing of Sunlenca as pre-exposure prophylaxis (PrEP) for men with topline data expected in late 2024 or early 2025.

Priority Health is acquiring 52,000-member Physicians Health Plan of Northern Indiana.

Public health, artificial intelligence and GLP-1s are on the agenda of the June 11-13 meeting in Las Vegas.

PDTs represent a shift in how treatment is delivered

Type 1 diabetes is an autoimmune disease that affects more than 2 million Americans.

An administration change in November could see the return of closed borders or increased protections for immigrants.