
CTTP is rare hereditary blood clotting disorder stemming from a disease-causing mutation in the ADAMTS13 gene. This gene plays a crucial role in producing the ADAMTS13 enzyme, responsible for regulating blood clotting.

CTTP is rare hereditary blood clotting disorder stemming from a disease-causing mutation in the ADAMTS13 gene. This gene plays a crucial role in producing the ADAMTS13 enzyme, responsible for regulating blood clotting.

Although the CDC predicted a decline in basic Medicare Part D premiums for 2024 due to the IRA, a recent report revealed an increase in premiums for Medicare Part D prescription drug plans in California, Florida, New York, Pennsylvania and Texas.

After two years of use, the twice-yearly Sunlenca remains active against HIV in patients with multi-drug resistant disease.

Topline results from week 16 of a phase 3b trial demonstrated the efficacy and safety of Tremfya across all skin tones.

Velsipity is the second S1P receptor modulator approved by the FDA to treat patients with moderate-to-severe ulcerative colitis. The wholesale acquisition cost is $6,164 for a 30-day supply.
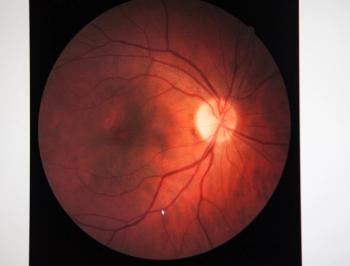
The FDA is currently reviewing a supplemental biologics license application for Vabysmo in patients with macular edema due to retinal vein occlusion. A decision from the FDA is expected in late 2023.

The IV formulation, a monthly 30-minute, weight-based dosing option, has a wholesale acquisition cost of $2,115 per vial. It will be available in the fourth quarter.
The dosage of Zoryve for children 6 to 11 is the same as for those over the age of 12. The list price is same for both pediatrics and adults — $825 per tube.
In a confirmatory trial, Exkivity did not meet the primary endpoint in treating patients with non-small cell lung cancer with EGFR exon 20 mutations. It will remain available while Takeda works with the FDA on withdrawal timing.

Ryzumi is expected to be available in the first half of 2024. Pricing will be available around the time of launch.

Iyuzeh is a preservative-free latanoprost product that can be stored at room temperature. It has a list price of $299 for a month’s supply.

By offering pre-conception, pregnancy, postpartum, and newborn interventions, as well as primary, wellness, and preventive care, this mobile health center is set to close the access gaps that have long affected the well-being of women and children in Texas.
Early results are expected late next year from the phase 1 trial, which is being funded by the National Institute of Allergy and Infecious Diseases, the Bill & Melinda Gates Foundation and Vir Biotechnology, a San Franciso biotech company focused on vaccines and infectious disease.
The FDA has granted priority review and assigned a Prescription Drug User Fee Act date of March 14, 2024, for resmetirom to treat adult patients with nonalcoholic steatohepatitis (NASH) who have liver fibrosis.

This average rate decrease will lower total premiums by an estimated $130 million.

The keynote speaker for the three-day meeting in Orlando is Sampson Davis, M.D., an emergency medicine physician in New Jersey, an inspirational speaker and co-author of three best-selling books.

The submissions to both the FDA and the EMA are supported by two phase 3 trials demonstrating Skyrizi achieved the primary endpoint of clinical remission.

Sandoz’s Tyruko, the first biosimilar to Biogen’s Tysabri, is expected to be available as soon as possible. Pricing has not yet been made available.

Izervay, which is administered with an intravitreal injection, is expected to be available within the two to four weeks.

Xdemvy is the first treatment that directly targets the mites involved in Demodex blepharitis. It is expected to be available by the end of August 2023 and will have a list price of $1,850 per prescription.
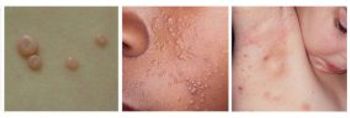
Ycanth is the first drug to treat children and adults with molluscum contagiosum, a viral skin infection. It will be available in September, but pricing information has not yet been released.

Results of Sanofi-sponsored study presented at ISPOR meeting also show lower hospitalization rates among app users.


More than two-thirds (68%) of the respondents to the survey expressed confidence that the COVID-19 is "behind us." An overwhelming majority (80%), though, indicated that annual COVID-19 vaccination will be needed for the foreseeable future.

Roctavian (valoctocogene roxaparvovec), the gene therpay for hemophilia A, gets nods as holding the most promise among the drugs in the pipeline.

Survey results published in today's Morbidity and Mortality Weekly Report show ventilation strategies were not reported by more than 51% of school districts. Of those that reported, low-tech and inexpensive continuous airflow, which can involve opening windows and using fans, was most popular.

Expenditures break down from managed care, out of pocket, investments and others. In 2022, the dominant share was held by managed care segment.

Amongst a few recommendations, Commonweath is encouraging how and how much we as payers, providers and consumers pay for primary care.

The Academy of Managed Care Pharmacy is throwing its support behind federal legislation that would create a Medicare benefit category for prescription digital therapeutics.