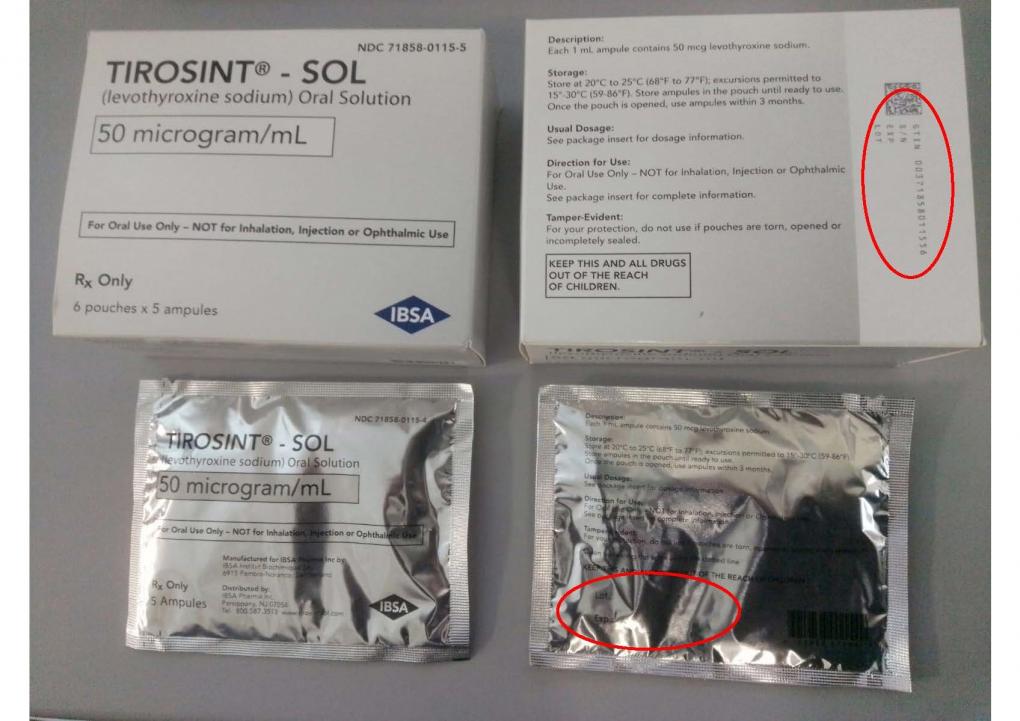
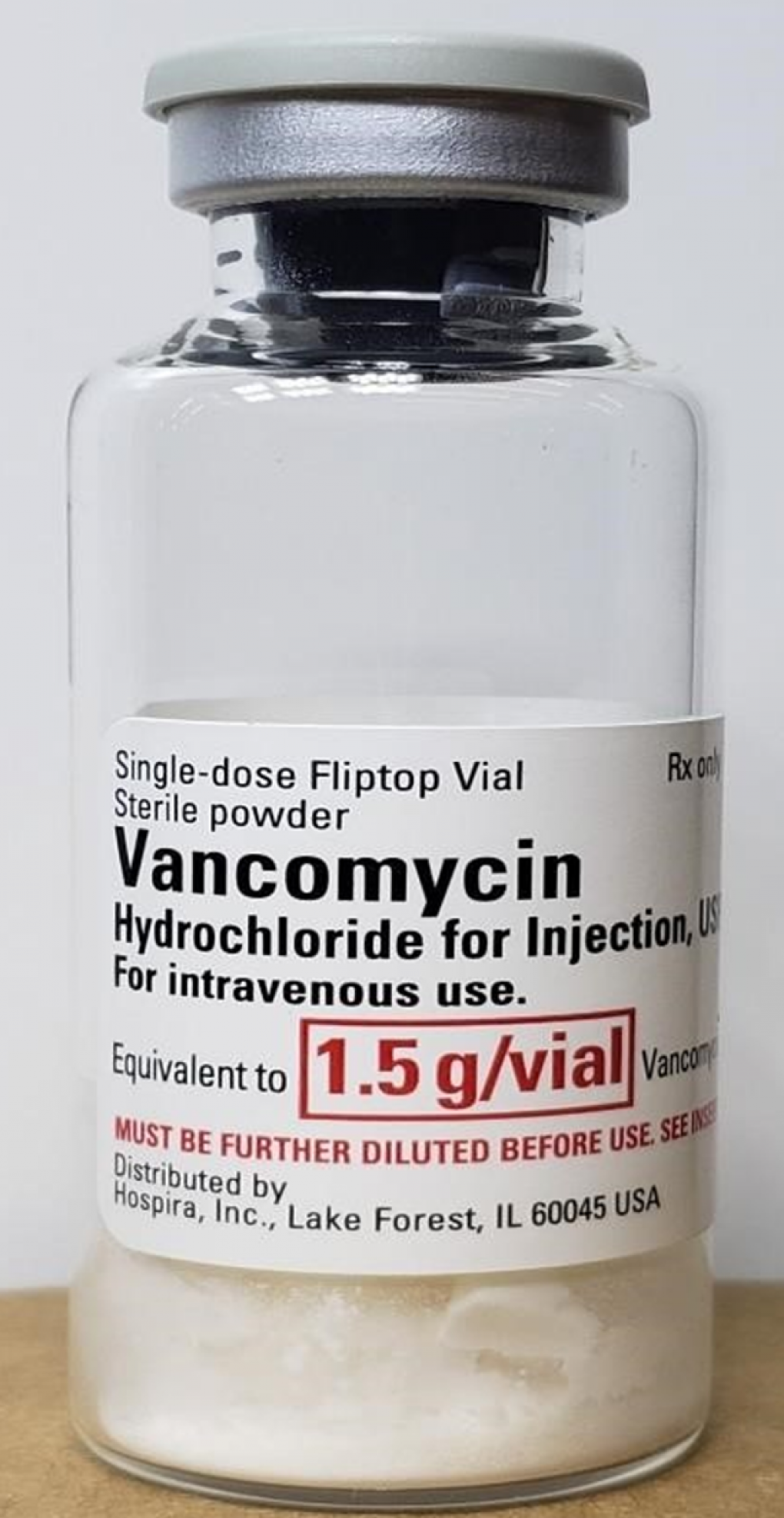
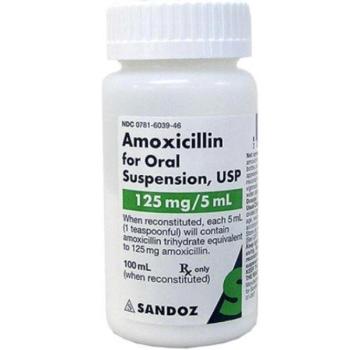
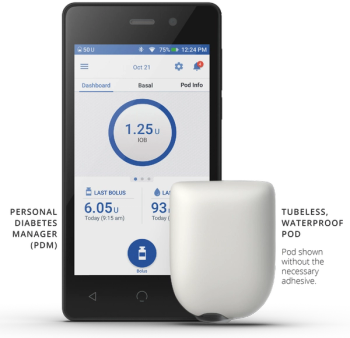
Among the top safety stories this year are Genentech’s warning to Ocrevus label, FDA’s update to GnRH agonists, a study about JAK-inhibitor side effects, FDA’s warning about Prolia in kidney disease and safety issues related to the potential Alzheimer’s disease drug lecanemab.