
A meta-analysis of more than 20 phase 3 trials found no clear survival differences by sex or age in metastatic NSCLC but reported better outcomes for Asian patients and continued underrepresentation of Black patients.

A meta-analysis of more than 20 phase 3 trials found no clear survival differences by sex or age in metastatic NSCLC but reported better outcomes for Asian patients and continued underrepresentation of Black patients.
Estimates show melanoma diagnoses continuing to climb in the United States, even as newer therapies continue to reduce deaths from advanced disease.

A survey finds that many dermatology patients struggle to understand skin cancer jargon, raising concerns about communication gaps during clinical visits.

The ADRIATIC trial showed that adding Imfinzi (durvalumab) improved both progression-free and overall survival of patients with limited-stage small cell lung cancer (LS-SCLC). However, the treatment increased costs by about $138,000 compared with usual care, highlighting concerns about affordability and financial impact.

Counties with more cultivated land and herbicide use had significantly higher melanoma rates, even after accounting for ultraviolet radiation and socioeconomic factors, a new geospatial study found.

In a study of more than 31,000 adults, an EHR-integrated patient-facing tool significantly improved identification of patients eligible for lung cancer screening by more accurately ascertaining smoking history.
A phase 3 trial offers new evidence that an immunotherapy drug may outperform chemotherapy for acral melanoma, a rare subtype long underrepresented in clinical research.

A new analysis suggests that Medicaid expansion was linked to lower mortality for patients with resectable non-small cell lung cancer, with survival benefits emerging gradually and persisting over time.
In a cohort study of 496 lung cancer survivors, 11.5% had a recurrence during a five-year follow-up period, and 5.6% had cancer outside the lungs.
Researchers found that less than half (43.8%) of the patients underwent surveillance imaging consistent with recommended guidelines.

A national survey of 157 patients with non-small cell lung cancer found that all groups prioritized extending life, but treatment preferences, including concerns about side effects and quality-of-life tradeoffs, differed across racial and ethnic groups.
Tumor infiltrating lymphocyte (TIL) therapy uses immune cells harvested directly from a patient’s tumor.


A study of more than 4,600 Medicare patients links treatment with Keytruda (pembrolizumab) to improved survival outcomes in adults 66 and older treated for metastatic non-small cell lung cancer.

The national five-year survival rate for lung cancer has climbed to nearly 30%, up from 18% from eight years ago, according to the American Lung Association.
Study participants who used the mPATH-Lung program were 60% more likely to undergo lung cancer screening, than particpants who did not use the program.
Although neuroendocrine tumors of the digestive system have been studied, far less is known about those that develop in the lungs, even as their incidence appears to be on the rise.

Most of the exposure to misinformation about cancer treatment occurs in conversations with family and friends and on social media.
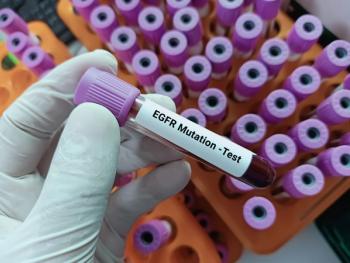
The FDA approved sunvozertinib, sold as Zegfrovy, in July 2025.

Patients with advanced non-small cell lung cancer were negatively affected by exposure to antibiotics, PPIs, and high-dose steroids at the start of treatment with Keytruda (pembrolizumab), French researchers found.

Disparities emerged in receipt of systemic therapy, particularly in lung cancer.
The study finds less than 1 in 4 go on to second-line treatment.
National registry study finds overall survival improved across the board after the introduction of immune checkpoint inhibitor (ICI) drugs, but gaps remained. Uninsured patients realized smaller gains compared with those with private insurance.
Patients using steroids, especially at high-doses, before taking immune checkpoint inhibitors for non–small cell lung cancer were more likely to have brain metastases.
The investigational agent, zipalertinib, received the breakthrough designation from the FDA in January 2022
Datroway (datopotamab deruxtecan) is the first TROP2-directed therapy approved for advanced lung cancer in the U.S.
The study is the first, to the researchers’ knowledge, to evaluate both survival and cost metrics across non-small cell lung cancer biomarker subtypes.

Published: November 23rd 2023 | Updated:
Published: April 19th 2023 | Updated:
Published: October 20th 2023 | Updated:

Published: April 26th 2023 | Updated:
Published: April 14th 2023 | Updated:

Published: May 26th 2023 | Updated: