Spinal Muscular Atrophy
Latest News
Latest Videos
CME Content
More News
A new method for delivering this treatment drug for spinal muscular atrophy offers an option for patients with advanced cases of the disease. But investigators found that the new method may also carry risks of mechanical failure and infection.
Small biotech companies such as Scholar Rock and Cytokinetics have treatments in late-stage trials for spinal muscular atrophy. Novartis and Biogen also have products in the pipeline.

The FDA has approved three targeted therapies for spinal muscular atrophy. They have hefty price tags so cost and affordability are live topics.
The phase 2 TOPAZ trial demonstrated treatment is safe and improves motor function in patients with SMA.
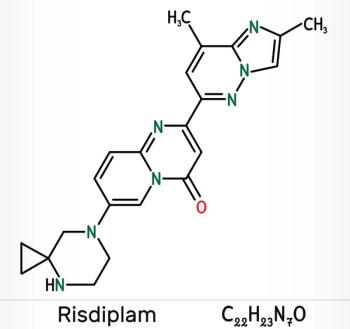
Patients treated with risdiplam at 12 months demonstrated significant improvement in survival and developmental milestones, with 19 of 21 (90%) infants able to survive without permanent ventilation and 7 (41%) infants able to sit without support for at least 5 seconds.


Adaptive Biotechnologies’ clonoSEQ gets third approval; FDA also acts on first at-home treatment for spinal muscular atrophy.