Blincyto is immuno-oncology therapy that targets CD19 surface antigens on B cells to treat patients with acute lymphoblastic leukemia. Its current wholesale acquisition cost price is $4,900.15 per vial.

Blincyto is immuno-oncology therapy that targets CD19 surface antigens on B cells to treat patients with acute lymphoblastic leukemia. Its current wholesale acquisition cost price is $4,900.15 per vial.

Imetelstat targets telomerase to inhibit uncontrolled proliferation of malignant stem and progenitor cells in myeloid hematologic malignancies.
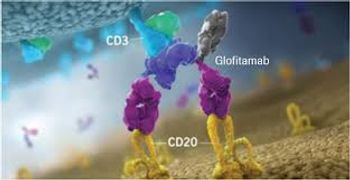
Columvi will cost about $350,000 for a fixed duration therapy with 12 treatments over eight and a half months.

More than 90% of cancer centers have experienced shortages of critical drugs. Erin R. Fox, Pharm.D., University of Utah Health, talks about why these shortages are happening and efforts that are being made to address them.
The median annual cost for new oncology medicines launched in 2022 was $260,000, up from $63,534 10 years ago, an IQVIA report finds.
The FDA has assigned an action date of Sept. 23, 2023. If approved, this would be the first frontline treatment advancement in decades for patients with advanced or recurrent endometrial cancer.

The combination of Lynparza and abiraterone reduced the risk of disease progression or death by 76% vs. abiraterone alone in patients with BRCA-mutated metastatic castration-resistant prostate cancer.

Ray Tancredi, VP of Walgreens mentioned in the last decade or so there have been many oncology drug approvals. Over time, the number of approvals decreased each year.

Medicare Advantage plans have been associated with increased screening rates. But managed care tactics and narrow networks may be a disadvantage for people needing care.
Out-of-pockets costs were higher among those using branded or more recently launched drugs.

Zynyx will be priced comparable to other PD-1 inhibitors currently available to treat metastatic or recurrent locally advanced Merkel cell carcinoma, a rare skin cancer.

Illuccix is for positron emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer.

Other news includes the launches of the first generic of an ulcer drug, the antipsychotic lurasidone, an extended-release version of topiramate for migraine, and additional strengths of the cancer drug lenalidomide. Additionally, Glenmark will distribute Cediprof's generic Adderall, and the FDA has accepted Amneal’s application for a generic naloxone nasal spray.
Niraparib and abiraterone acetate plus prednisone has potential to address unmet need for patients with BRCA-positive metastatic castration-resistant prostate cancer.

BMS is seeking an indication for Opdivo as monotherapy to treat patients with stage IIB or IIC melanoma after surgery. The FDA has assigned a Prescription Drug User Fee Act date of Oct. 13, 2023.

Patients who have had liver problems in the past may be at risk of liver damage from Sprycel, which is used to treat patients with chronic myeloid leukemia.

Phase 3 data showed that the combination resulted in a 37% reduction in risk of disease progression or death in men with advanced prostate cancer.

Conversion from accelerated to regular approval was based on long-term outcomes from a phase 1 trial, which demonstrated an overall response rate of 45.4%.
Gastroesophageal reflux disease is a risk factor for what is now the more common type of esophageal cancer, adenocarcinoma. Immunotherapy is coming on strong as a treatment.
Trodelvy has been recommended as a preferred treatment for metastatic HR+/HER2- breast cancer by the National Comprehensive Cancer Network.

Dosing has been lowered to reduce the risk of liver damage in patients taking Turalio, which is approved to treat patients with a rare tumor that affects the joints.

The collaboration between Cost Plus Drugs and OncoPower provides access to generic medications for patients with cancer integrated with OncoPower’s Pill Reminder tool.
Elacestrant, now with the brand name Orserdu, is an oral selective estrogen receptor degrader (SERD), which works by blocking the effects of estrogen on hormone receptor-positive breast cancer cells.

Jaypirca is the first BTK inhibitor specifically approved for patients with mantle cell lymphoma previously treated with a covalent BTK inhibitor. It has a wholesale acquisition cost of $21,000 per 30 days of therapy.

This approval marks the fifth indication for Keytruda-based regimens in non-small cell lung cancer and the 34th indication in the United States.