Oncology
Latest News

Latest Videos
CME Content
More News

A new survey by Johnson & Johnson revealed that oncologists are overwhelmed by the rapid pace of innovation in cancer care, with many calling for better tools, education and collaboration to help integrate new treatments and technologies into everyday practice.
The first CAR-T cell therapy was developed and approved by the FDA in 2017 to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). Since then, six additional CAR-T cell therapies have been introduced to the market. Four of the seven are approved for B-cell lymphomas.
In January 2023, the Brukinsa, a next-generation BTK inhibitor, received FDA approval for chronic lymphocytic leukemia (CLL).

Experts at City of Hope, Simon Nazarian and Nasim Eftekhari, discussed the transformative potential of AI in cancer care.

Oncologists should ask in advance about what young patients and their parents want to be told about changing prognoses, a new qualitative study finds

In a statistical analysis, Brukinsa resulted in fewer cases of disease progression or death and resulted in lower overall healthcare costs than Imbruvica in patients with chronic lymphocytic leukemia.

One particular application of AI on the rise at City of Hope is the development of a large language model (LLM) made specifically for oncology.

About 6% of pregnancy-related cancers are Hodgkin lymphoma, and 5% are non-Hodgkin lymphoma. Leukemias in pregnancy are rarer, accounting for 1 in 10,000 pregnancies. The most common pregnancy-related leukemias are acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML).

Experts at City of Hope, a cancer research and treatment center, believe that AI tools will continue to improve precision medicine, streamline patient care and increase access to clinical trials.

In a February 2025 Time magazine cover story, Jamie Ducharme reports on this atypical and disquieting trend. Ducharme cites a 79% increase in early-onset cancer diagnoses and a 28% increase in cancer-related deaths in this age group from 1990 to 2019.

In a real-world study, researchers found this data by comparing treatment patterns, healthcare resource utilization, and costs between patients receiving CAR T-cell therapy and those treated with the non-CAR T standard of care.

The study included 145 patients who were prescribed Calquence, Imbruvica, or Venclexta to treat CLL or SLL and filled the medications through the IHSSP at Vanderbilt University Medical Center.

Esophageal squamous cell carcinoma accounts for approximately 90% of all esophageal cancer cases. Projections estimate that by 2040, there will be approximately 957,000 new cases of esophageal cancer worldwide, marking a nearly 60% increase from 2020, according to BeiGene.

Although CAR T-cell therapies have been found effective against certain difficult-to-treat malignancies, they are not devoid of safety concerns.

Cedars-Sinai was one of 12 among 172 adult transplant centers in the U.S. with a one-year patient post-transplant survival rate above the expected outcome. In 2024, the survival rate for Cedars-Sinai was 90% versus the expected rate of 68% to 83%.
Treosulfan (Grafapex) was approved in Canada in June 2021 for the same use and is marketed under the brand name Trecondyv.

The drug received accelerated approval in October 2017 as monotherapy for adults with MCL who have received at least one prior treatment. The FDA simultaneously converted the conditional approval to full approval for this indication.

The investigational drug, called PMD-026, is currently in phase 2 clinical trials for the treatment of breast cancer.
The antibody-drug conjugate (ADC), marketed by ADC Therapeutics under the brand name Zynlonta, received accelerated approval in April 2021. Full approval is contingent upon positive results from confirmatory trials.
Gastric cancer, commonly known as stomach cancer, is the fifth most common cancer globally and the fifth leading cause of cancer-related deaths.

The FDA has already made more than a dozen cancer drug approval decisions this year and more expected in the next several months and in early 2025, according to Kaelyn Boss, who gave an oncology drug pipeline talk at the 2024 AMCP Nexus meeting this week.

Time can be especially precious for people with advanced cancer. Researchers and clinicians are beginning to consider “time toxicity” as another aspect of treatment that burdens patients and their loved ones.
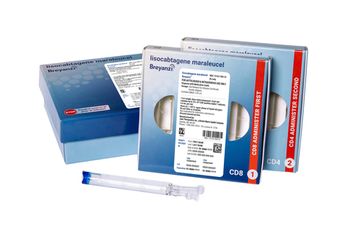
Breyanzi is a CAR T-cell therapy now approved for four subtypes of non-Hodgkin lymphoma.
Verastem is studying the combination of avutometinib and defactinib to treat low-grade serous ovarian cancer in patients with KRAS-mutations. The company plans to complete the new drug application in the second half of this year.
Tarlatamab — now with the brand name Imdelltra — is the first approved bispecific antibody for a solid tumor.