
ASTRO's new guidelines enhance radiation therapy strategies for gastric cancer, improving treatment outcomes and patient care across various stages of the disease.

ASTRO's new guidelines enhance radiation therapy strategies for gastric cancer, improving treatment outcomes and patient care across various stages of the disease.
Novo Nordisk has a factor VIII mimetic in a phase 3 trial, and a Chinese company is forging ahead with its gene therapy product.
Researchers reveal ELI-002 2P cancer vaccine shows promise in boosting immune responses for colorectal and pancreatic cancer patients, enhancing survival rates.
According to the American Cancer Society, about 22,000 new esophageal cancer cases were diagnosed in 2025 in the U.S., and over 16,000 deaths were attributed to the disease. Risk factors for this carcinoma include obesity, gastroesophageal reflux disease (GERD) and Barrett’s esophagus.
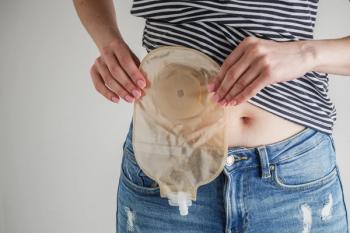
Colorectal cancer screening rates in young adults rise with effective outreach strategies, highlighting the importance of FIT kits for early detection.

H. pylori infection significantly contributes to stomach cancer risk, but effective screening and treatment can prevent millions of cases globally.
Private equity acquisitions have increased rapidly across all aspects of healthcare, with gastroenterology practices experiencing the highest share. In the U.S., approximately 13% of gastroenterologists work in practices owned by private equity groups.


Current treatment options have improved survival rates but have not provided a cure. Eventually, initial therapies stop working, and many patients relapse.

Financial toxicity can affect patient outcomes and quality of life. For example, a patient may forgo treatment or medications to save money, or they may incur high medical debt or go into bankruptcy to pay for medical care.
The first CAR-T cell therapy was developed and approved by the FDA in 2017 to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). Since then, six additional CAR-T cell therapies have been introduced to the market. Four of the seven are approved for B-cell lymphomas.

Agents that target NaV1.8, a voltage-gated sodium channel, are showing promise. The FDA approved one of them, Journavx (suzetrigine) in January 2025.
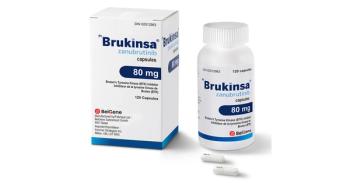
In January 2023, the Brukinsa, a next-generation BTK inhibitor, received FDA approval for chronic lymphocytic leukemia (CLL).

Several drugs in development would block neonatal fragment crystallizable receptor (FcRn) as a way of reducing the autoantibodies that cause myasthenia gravis.

About 6% of pregnancy-related cancers are Hodgkin lymphoma, and 5% are non-Hodgkin lymphoma. Leukemias in pregnancy are rarer, accounting for 1 in 10,000 pregnancies. The most common pregnancy-related leukemias are acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML).

In a February 2025 Time magazine cover story, Jamie Ducharme reports on this atypical and disquieting trend. Ducharme cites a 79% increase in early-onset cancer diagnoses and a 28% increase in cancer-related deaths in this age group from 1990 to 2019.

In a real-world study, researchers found this data by comparing treatment patterns, healthcare resource utilization, and costs between patients receiving CAR T-cell therapy and those treated with the non-CAR T standard of care.

The study included 145 patients who were prescribed Calquence, Imbruvica, or Venclexta to treat CLL or SLL and filled the medications through the IHSSP at Vanderbilt University Medical Center.
Drugs in late-stage development may mean competition on price for two Pfizer medications that, by some accounts, are the most expensive cardiac medications in history.

Although CAR T-cell therapies have been found effective against certain difficult-to-treat malignancies, they are not devoid of safety concerns.

Cedars-Sinai was one of 12 among 172 adult transplant centers in the U.S. with a one-year patient post-transplant survival rate above the expected outcome. In 2024, the survival rate for Cedars-Sinai was 90% versus the expected rate of 68% to 83%.
Treosulfan (Grafapex) was approved in Canada in June 2021 for the same use and is marketed under the brand name Trecondyv.

The drug received accelerated approval in October 2017 as monotherapy for adults with MCL who have received at least one prior treatment. The FDA simultaneously converted the conditional approval to full approval for this indication.

The investigational drug, called PMD-026, is currently in phase 2 clinical trials for the treatment of breast cancer.
The antibody-drug conjugate (ADC), marketed by ADC Therapeutics under the brand name Zynlonta, received accelerated approval in April 2021. Full approval is contingent upon positive results from confirmatory trials.

It is known that patients with active IBD have an increased risk of preterm birth, slowed fetal growth, low birth weight, hypertensive disease of pregnancy, and cesarean birth. On the other hand, conception during disease remission results in similar relapse rates as for people with IBD who are not pregnant.

Previous studies have found no association between vaccination and MS in adults. To evaluate the association between vaccinations and pediatric-onset MS, researchers conducted a retrospective case-control study using data from the Bavarian Association of Statutory Health Insurance Physicians database.

Published: February 24th 2022 | Updated:

Published: January 27th 2022 | Updated:
Published: April 10th 2022 | Updated:

Published: September 18th 2022 | Updated:

Published: March 15th 2024 | Updated:

Published: November 3rd 2022 | Updated: