
Asthma becomes more prevalent in females than males in puberty. Researchers have hypothesized that sex hormones may play a significant role in the manifestation of asthma symptoms.

Asthma becomes more prevalent in females than males in puberty. Researchers have hypothesized that sex hormones may play a significant role in the manifestation of asthma symptoms.
Bispecific antibody therapies are also in development.
Six biologics are on the market and more are in the pipeline. Biologics target the inflammatory cytokines involved in the pathogenesis of asthma.
University of North Carolina researchers report that interleukin-13, responsible for inflammation and severe asthma symptoms, may have properties that fend off the SARS-CoV-2 virus that causes COVID-19.

But researchers find that fewer than 1 in 4 children and young adults had follow-up visits.

California’s “in lieu of services” (ILOS) program includes asthma remediation services such as dehumidifiers, improved ventilation and mold removal.

Across the globe, new drugs — injections, topical and oral — are being developed to help patients with atopic dermatitis.
Genentech researchers identify oncostatin M as a protein that may be responsible for the severe airway inflammation seen in patients with noneosinophilic asthma.

Real-world study conducted in Japan shows Dupixent (dupilumab) reduced the number of annual severe asthma exacerbations by 53%. There were some reports of adverse events associated with high eosinophil levels in patients who switched to Dupixent after taking a different biologic.
Xolair (omalizumab), Fasenra (benralizumab), Dupixent (dupilumab) and Nucala (mepolizumab) can now be self-administered.
The race is on to be the first-approved Bruton tyrosine kinase inhibitor. Meanwhile, trials are underway to test statins and metformin as treatments for multiple sclerosis.
Getting boosted is now the rule, not the exception, when it comes to COVID-19 vaccination recommendations. The CDC now recommends that everyone, ages 12 and older, get a dose of an mRNA COVID-19 vaccine, either Pfizer’s or Moderna’s. For people who have gotten the two-shot Pfizer or Moderna series, the booster is their third jab. For those who got the single-dose Johnson & Johnson vaccine, it is the second.
For now, only the Pfizer and Moderna vaccines have full FDA approval, but many candidates are in phase 2 or 3 trials.

The setback for GlaxoSmithKline comes amid a multicompany race to develop a vaccine against respiratory syncytial virus infections.

Pyrukynd, developed by Agios Pharmaceuticals, is a PK activator and the first in this drug class. The company is also awaiting approval by the European Medicines Agency, and a decision is expected by the end of 2022.

Asundexian is an oral factor XIa inhibitor currently under phase 2 trials for potential secondary thrombosis prevention in patients with non-cardioembolic ischemic stroke, atrial fibrillation, or recent myocardial infarction.
The oral formulation of the muscle relaxant was also approved as a treatment for spasticity associated with spinal cord injuries.
Pfizer and BioNTech initiated an application yesterday (Feb. 1) for emergency use authorization (EUA) of their COVID-19 vaccine for children ages 6 months through 4 years.

The dual orexin receptor antagonist (DORA) from a Swiss drugmaker will be available in May 2022.

The Janssen drug is the only DOAC available in an oral suspension, which facilitates pediatric weight-based dosing.
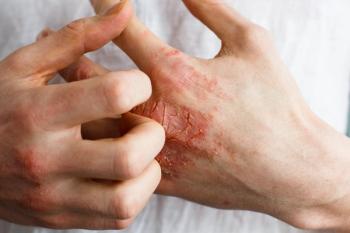
Leo Pharma's Adbry (tralokinumab-ldrm) is the second, FDA-approved biologic for atopic dermatitis. Dupixent (dupilumab) was the first.

Leqvio (inclisiran) is the first small interfering RNA therapy approved to lower LDL cholesterol in certain adults at risk for life-threatening cardiovascular events.