Oncology
Latest News
CME Content


Two new studies look at the financial hardships faced by those with a cancer diagnosis. Authors of both suggest there is a need for improving health insurance coverage.
A phase 3 trials showed Tivdak demonstrated a 30% reduction in the risk of death. The U.S. list price is $6,599 per 40 mg single dose vial.

Ojemda will have a wholesale acquisition cost of $33,916 for a 28 day supply and will be distributed through specialty pharmacies Biologics by McKesson and Onco360.

Lutathera is a radiotherapeutic that is now approved for patients 12 years and older neuroendocrine tumors in the pancreas or gastrointestinal tract.
ImmunityBio’s Anktiva is an antibody cytokine fusion protein approved to treat patients with non-muscle invasive bladder cancer.
The FDA is currently reviewing tarlatamab for small-cell lung cancer with a review date in June and datopotamab deruxtecan for non-small cell lung cancer with a review date in the fourth quarter.

New study finds trial enrollment was highest at NCI-designated cancer centers. Researchers said more needs to be done to enroll patients from community cancer sites.

Between 2011 and 2020, 45.6% of new cancer drugs were approved through the accelerated approval pathway, according to a new report from the American Association of Cancer Research.

Datopotamab deruxtecan, which is being reviewed to treat adults with HR-positive, HER2-negative breast cancer, has an FDA target date in the first quarter of 2025. Datopotamab deruxtecan also is under FDA review for non-small cell lung cancer.
FDA officials indicated that the confirmatory trial for odronextamab for patients with follicular lymphoma and diffuse large B-cell lymphoma should be under way before resubmission.
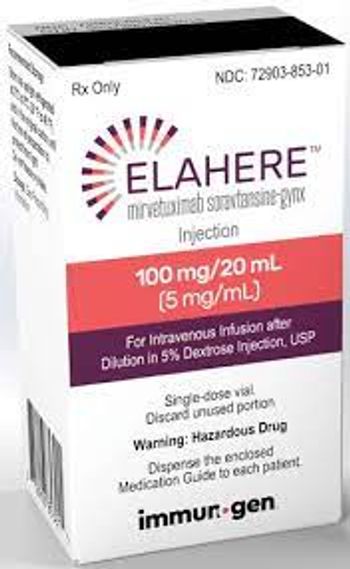
The full approval was based on the confirmatory phase 3 MIRASOL trial, which showed that Elahere resulted in a 33% reduction in risk of death and a 35% reduction in the risk of cancer progression.

The accelerated approval of Iclusig for patients with acute lymphoblastic leukemia and with the Philadelphia chromosome was based on a surrogate endpoint. Iclusig has a list price of $20,831 for 30 tablets.

The FDA has assigned a goal date of Aug.13, 2024, for Lymphir to treat patients with relapsed or refractory cutaneous T-cell lymphoma.
Tevimbra will be available in the second half of this year and is indicated as a second-line treatment of patients with esophageal squamous cell carcinoma, which is an aggressive cancer.
Breyanzi is the first CAR-T therapy to treat adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma.
Both Wyost and Jubbonti are interchangeable for the reference products and are approved for all of the same indications

ONC201 (dordaviprone) displayed “well tolerated and durable and clinically meaningful efficacy in Histone 3 (H3) K27M mutant-diffuse midline glioma DMG.”

Rybrevant is indicated to be used in combination with chemotherapy as a first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer.
The FDA has set an action in December 2024 for tislelizumab — now with the brand name Tevimbra — for metastatic gastric or gastroesophageal junction cancers.

The FDA is reviewing Epkinly to treat relapsed or refractory follicular lymphoma. The target date is in August 2023.
Regulators indicated that the confirmatory study did not confirm Pepaxto’s clinical benefit and that there was no evidence of safety or efficacy.

In this month’s episode of Tuning In to the C-Suite, Briana Contreras, an editor of Managed Healthcare Executive, got to chatting with Sanjula Jain, Ph.D., chief research officer at Trilliant Health.
Research results reported in the journal Science today add to the evidence that “immunogenetics” likely influences people’s susceptibility to developing lung cancer, especially among smokers.
Patients with multiple myeloma who switched from weekly to biweekly dosing of Tecvayli were able to maintain their response with fewer infections.