
Many vitiligo studies don't include enough people with darker skin types, which often leads to missing key demographic details.

Many vitiligo studies don't include enough people with darker skin types, which often leads to missing key demographic details.
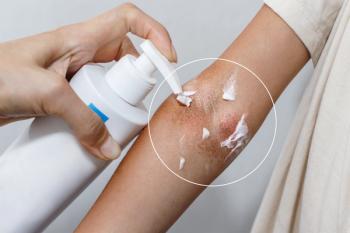
These results were found in the TRuE-AD3 study that was presented at the Society for Pediatric Dermatology meeting earlier this month, revealing the latest round of data collected in the TRuE-AD1 and TRuE-AD2 series of studies.

Although AD is a common condition, it’s impact on sexual function and reproductive health is not well understood. In addition, many women with AD are undertreated during pregnancy due to concerns about medication side effects.
According to a Frontiers in Neurology study, about .3% of people have PD, but it’s more common in older adults: 1% of those over 60 and 3% over 80.

Researchers who conducted the study, of the Universities of California in San Francisco and Berkley, suggest that cutting down on sodium could be a more cost-effective and low-risk way to help manage AD.

The new 2 mL autoinjector offers a more convenient option for adult patients by reducing the number of required injections by half compared to the pre-filled syringe.
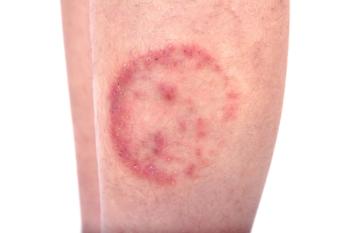
Experts are urging healthcare providers to be aware of these two new forms of ringworm or jock itch, known as Trichophyton mentagrophytes type VII (TMVII) and Trichophyton indotineae or T. indotineae.

In a recent American Academy of Dermatology survey, it was revealed that 52% of Gen Z adults ages 18 to 26 are unaware of key sunburn risks, such as increased skin cancer risk and premature aging.

Patients with AD often experience different degrees of itch and lesions, with some expressing more severity than others.
The FDA has approved six with dermatologic indications and more are in late-stage trials.

Joseph Zabinski, PhD, MEM, vice president, head of commercial strategy and AI, OM1, chatted with MHE editors on the significance of patient acceptance in AI adoption in healthcare, overall and in the dermatology space, stressing trust and transparency.

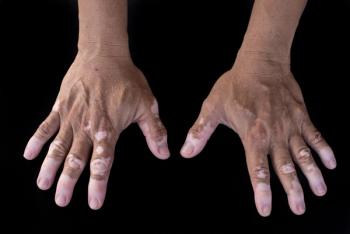
Researchers of a study conducted a double-blind intervention that aimed to assess the effectiveness and tolerance of a new approach for vitiligo treatment using meso-microneedling with 5% N-acetylcysteine (NAC) ampoules and topical 4.7% NAC, compared to microneedling alone.
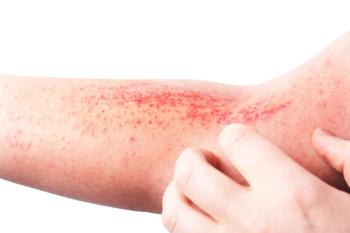
Adults and children with atopic dermatitis experience worse anxiety and depression as disease becomes more severe.

Povorcitinib, an oral JAK1 inhibitor, shows promise in treating prurigo nodularis, a chronic, inflammatory skin disease.

Ruxolitinib cream showed positive results in children 2- to 11-years-old with mild to moderate atopic dermatitis (AD), according to data of a poster presented at the American Academy of Dermatology Annual Meeting in San Diego from March 8 to 12.
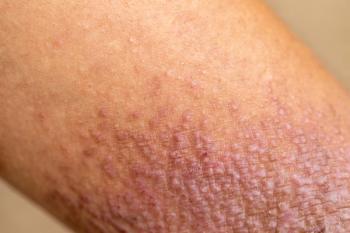
Physicians switch to Opzelura (ruxolitinib) when other therapies fail to help patients with atopic dermatitis, according to a new analysis presented at the annual meeting of the American Academy of Dermatology.

John Barbieri, assistant professor at Harvard Medical School, is looking forward to the upcoming 2024 American Academy of Dermatology meeting, which runs from March 8-12 in San Diego. Barbieri is particularly showing interest in late-breaking abstracts and trial data and hoping to see cutting-edge information from pivotal clinical trials.
Amtagvi was approved to treat patients with advanced melanoma. It is a one-time cell therapy that will be administered at authorized treatment centers and have a wholesale acquisition cost of $515,000.

This makes CSU treatment the sixth approved use globally.
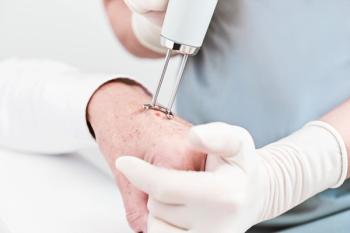
SkinCure Oncology has not yet submitted its data to the FDA for marketing clearance, however once cleared, the new devices will use hybrid laser-generated confocal and photoacoustic imaging in a single fused volumetric image.

The biopharmaceutical company developed VTAMA cream as a once-daily and steroid-free topical cream for both acute treatment and long-term management of AD.
The molecule, DPT0218, was detected during preclinical animal trials.

A recent survey by the American Academy of Dermatology showed that only 13% of Americans plan to use sun protection when going for walks or hikes in cold weather.

New acne guidelines from the American Academy of Dermatology recommend Winlevi (clascoterone), a topical treatment, and Seysara (sarecycline), a narrow-spectrum tetracycline, but qualify the recommendation as conditional because of concerns that the high cost may affect equitable access to treatment,

A review of published research identified only five studies. They showed that telemedicine, particularly for follow-up care, was as effective as in-person care in many respects