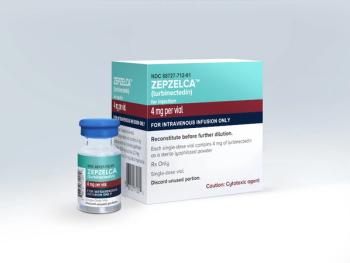
Two new subsections have been added to the warning and precautious section of Zepzelca’s label to address serious adverse events that were identified during post-market monitoring.

Two new subsections have been added to the warning and precautious section of Zepzelca’s label to address serious adverse events that were identified during post-market monitoring.

Drew Mihalyo of Delta Care Rx discusses the company’s pass-through pharmacy pricing model.
Treatment with Zegalogue for severe hypoglycemia has the potential to save costs and reduce emergency department visits and hospitalizations.

A proof-of-concept study showed that patients can be successfully switched from Cosentyx to Taltz with similar outcomes and lower costs.

Karthik Ganesh, CEO of EmpiRx Health, discusses how its technology and clinical focus provide strategies for patient management that results in lower costs for clients.

The PBM has removed 20 products and added a half dozen new therapies, including several generics. The changes are effective April 1, 2022.
Medicare beneficiaries without subsidies were 35% to 50% less likely to fill their prescriptions for drugs used to treat cancers, immune system disorders, and high cholesterol.

The agency has extended the review period to address pending inspection classification at third-party secondary packaging and labeling facility. The new PDUFA date is July 14, 2022.
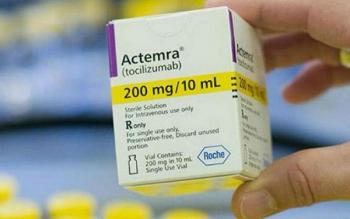
Genentech also announced progress in meeting the supply challenges associated with Actemra, which is currently available under an emergency use authorization.

The FDA authorizes second COVID-19 booster and approves a higher does of Ozempic, Cabenuva for adolescents with HIV, a therapy for rare seizer disorder, and an oral testosterone replacement. The agency issues a complete response letter for a therapy for anemia related to chronic kidney disease, and an advisor committee votes down a drug for ALS.
Insurers need a better understanding of patients’ adherence and discontinuation of medication for primary biliary cholangitis, according to AllianceRx Walgreens Prime study.

The FDA’s final decision on AMX0035 is expected by June 29, 2022

AllianceRx Walgreens Prime study has found that patients taking Ocaliva may not be adherent to their treatment regimen for rare liver disease.

A disconnect exists between payers and patients with rare diseases about including patient input in the formulary process for orphan drugs.

Antares Pharma plans to launch Tlando, which doesn’t require dose titration, in the second quarter of 2022.

Prior authorization and step therapy can impact compliance and outcomes, according to a survey by the Pharmaceutical Research and Manufacturers of America.

Keytruda gets another indication, the first targeted therapy for advanced prostate cancer is approved, BMS snags approval for Opdualag, the FDA misses PDUFA date for Reblozyl and issues CRLs for sintilimab and Natpara.

The FDA has asked for an additional clinical trial with overall survival as the endpoint.

The new code will be effective April 1, 2022.
The study aims to generate data related to Tremfya in people of color who have plaque psoriasis.
But there is no significant difference in rate of hospitalization for major bleeding when Eliquis is compared with Xarelto or warfarin.

Teplizumab is being evaluated to delay type 1 diabetes. The PDUFA date is August 17, 2022.

FDA approves first generic of Symbicort for asthma and COPD and first therapy for rare seizure disorder, Rinvoq and Lynparza get nods for new indications, the agency approves a new delivery system for Alzheimer’s therapy, regulators extend review for Opzelura for skin condition and issue CRL for Fasenra for chronic rhinosinusitis.

Del Doherty, co-founder of Prodigy Rx, discusses how the PBM aims to give payers control so they can lower costs and can improve clinical and return-to-work outcomes.

Amneal is of 35 global companies selected to manufacture and commercialize generic version of COVID-19 treatment Paxlovid.

ICER analyzed two therapies — Cosela and plinabulin — to prevent chemotherapy-induced neutropenia and other myelosuppressive effects. Both moderately increased quality-adjusted life-years.
ICER also reviewed, plinabulin, which is development chemotherapy-induced neutropenia (CIN) and would achieve common thresholds for cost-effectiveness if priced between $1,100 and $1,600 per dose.

Allan Coukell, Civica’s senior vice president of public policy, discusses how the generic manufacturer is disrupting the market for insulins.

Evio Pharmacy Solutions plans to provide biosimilar alternatives for autoimmune and cancer therapeutics.

After a phase 3 trial showed that Cabometyx/atezolizumab did not improve overall survival in patients with hepatocellular carcinoma, Exelixis officials have said they won’t be submitting an NDA for untreated patients with advanced liver cancer.