
In a study, both common and rare HMGCR variants were associated with an increased risk for cataract.

In a study, both common and rare HMGCR variants were associated with an increased risk for cataract.

In COVID-19 news, the FDA has declined an EUA request for Zyesami and Pfizer has submitted NDA for Pavlovid. The regulatory agency has accepted several applications, including an sBLA for Eylea in diabetic retinopathy and the NDA for palovarotene for a rare genetic disorder. The FDA also issued a CRL for a UTI therapy and extended the review of diabetes therapy. And finally, Gilead has resubmitted its twice-year HIV therapy.

Effective July 1, 2022, CVS Caremark has removed 16 drugs and added 14 to its Standard Control Formulary.
Sanofi has lowered the out-of-pocket cost for those without insurance of its insulins to $35 for a 30-day supply through the company’s savings program.
If approved, lenacapavir would be the first HIV-1 treatment administered twice-yearly.

Labels now warn that buprenorphine products that dissolve in the mouth can cause dental problems. Buprenorphine is used to treat opioid use disorder and pain.

FDA approves tumor-agnostic cancer therapy, Clovis pulls Rubraca indication, Purdue launches opioid reversal, FDA plans advisory committee meeting for Xphozah, and Manarini and AbbVie submit applications.
Benefits leaders surveyed in PSG’s Trends in Drug Benefit Design Report say the importance of the member experience has increased since the start of the pandemic.

Increased demand for certain medications used to treat hospitalized patients with COVID-19 had a significant impact on medication supply last year.

The company will take no profit, which is part of the company’s bankruptcy filing and settlement with states.
A study in the journal Blood has found that the cost-effectiveness of the Polivy treatment regimen used to treat patients with diffuse large B-cell lymphoma would decrease if the five-year progression-free survival decreased.

Committee members said the data from the two trials submitted didn’t support efficacy of Nuplazid in Alzheimer’s patients. The PDUFA target date is Aug. 4, 2022.

In COVID-19 news, the FDA expanded emergency use of the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 Vaccine for children down to 6 months of age. The FDA also approved Skyrizi for Crohn’s disease, Olumiant for alopecia, as well as two rare disease therapies: Imcivree for obesity associated BBS and Amvuttra for neuropathy associated with rare disease. Additionally, FDA extended the PDUFA date Brukinsa.

Walgreen’s patient insights and technology capabilities, along with partner healthcare companies, will aim to engage broader and more diverse communities for participation in clinical trials.

The Federal Trade Commission said that paying or accepting rebates or fees in exchange for excluding lower cost drugs on formularies could be considered commercial bribery.

Dogged by controversy, the new Alzheimer’s drug has not fared well since its approval by the FDA a year ago.
A literature review by AmerisourceBergen's Xcenda identifies a link between high cost sharing and patient nonadherence to medication regimens.
Genentech’s anti-amyloid crenezumab did not slow or prevent cognitive decline in people with early-onset Alzheimer’s disease.

Joe Murad, president and CEO of the pharmacy benefit manager WithMe Health, talks about how consumer-driven healthcare requires access, transparency, and easier navigation.

Researchers writing in Health Affairs have found that new patents for updated devices have delayed generic competition in asthma and COPD treatments.

A generic is available for one, and Centers for Medicare & Medicaid Services has removed two others from Medicare Part D coverage.

Amvuttra is expected to launch in July, and the developer, Alnylam, plans to make this available through value-based agreements.

Olumiant is the first systemic therapy available to treat alopecia.

In February 2022, the FDA had issued a complete response letter in order to inspect a new third-party packaging and labeling facility.

If approved, beti-cel will be the first potentially curative gene therapy for people with beta-thalassemia who require regular red blood cell transfusions. The PDUFA date is Aug. 19, 2022.

In COVID-19 news, an FDA advisory committee recommends EUA for Novavax’s vaccine. The FDA has also granted approvals to Dupixent for children as young as 6 month with atopic dermatitis and to the biosimilar Riabni for rheumatoid arthritis. An advisory committee has also recommended approval for two gene therapies, one for a rare neurogenerative disease and another for a blood disease.

If approved, elivaldogene autotemcel will be the first gene therapy to address the underlying genetic cause of cerebral adrenoleukodystrophy, a rare disease that affects young boys.
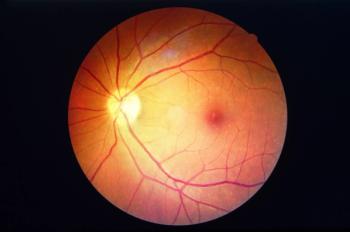
A biomarker used in kidney disease screening has potential to identify people with sight-threatening diabetic retinopathy.

Two studies conducted by AllianceRx Walgreens Prime call attention to the importance of adherence for reducing risk of negative health outcomes and financial costs.

Perfluorohexyloctane prevents excessive tear evaporation and has the ability to restores tear film balance. A new drug application is expected to be filed this year.