The FDA has requested Acadia conduct an additional trial of Nuplazid to support an indication in patients with Alzheimer’s disease.

The FDA has requested Acadia conduct an additional trial of Nuplazid to support an indication in patients with Alzheimer’s disease.

Vabysmo is the first injectable bispecific antibody to treat wet age-related macular degeneration and diabetic macular edema. Some recent data suggests that the injections can be administered less often than first indicated with no loss of efficacy.

Coherus’ Cimerli has been approved to be interchangeable for all five indications, including age-related macular degeneration and diabetic retinopathy. It will be available in early October 2022.

Investigators found that socioeconomic and financial barriers can affect patients’ access to oral cancer medications. They suggest that addressing these barriers could improve adherence.

Prime has removed six drugs from its formularies because generics are now available. One has been removed because it has been discontinued.
Optum has teamed up with Sanofi to provide savings cards for six insulins through the Optum Store.

A small study finds that an adeno-associated virus gene therapy eliminated both spontaneous bleeding and the need for factor IX prophylaxis in patients with hemophilia B.

The FDA has approved lupus therapy for children and allows importation of contrast medium. The agency has accepted applications for several therapies, including for a Tysabri biosimilar, a therapy for a genetic form of ALS, and an immunotherapy for bladder cancer, as well as for Enhertu in low HER2 breast cancer. Additionally, AbbVie seeks additional indication for Rinvoq.

Kelly Stanton, director of quality at Qualio, talks about the impact on patients of prescription drug recalls.

This follows a move by Bayer to import Ultravist with non-U.S. labeling also to address pandemic-related shortages of iodine-containing contrast media used in imaging procedures.

FDA approves Xalkori for rare tumor, as well as Opzelura for skin conditions. The agency has accepted applications for a supplemental indication for the biosimilar Hyrimoz and pegcetacoplan for advanced macular degeneration. Additionally, Acadia submits NDA for trofinetide.

David Lassen, Pharm.D., chief clinical officer at Prime, discusses a pilot program that used a predictive model to identify patients at high risk of overusing opioids.

The organization claims that direct and indirect remuneration fees and other clawback programs amount to a breach of contract.

Navitus senior vice president and chief pharmacy officer Brent Eberle talks about how partnering with CivicaScript fits into its cost-plus model.
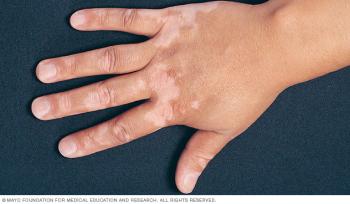
Opzelura is first therapy approved to treat patients with vitiligo, a disease that causes the loss of skin color.
BMS warns about the risk of extramedullary hematopoiesis, a rare complication in which the production of blood cells occurs outside of the bone marrow.

In COVID-19 news, the FDA grants EUA for Novavax’s COVID-19 vaccine. The agency approved a novel diagnostic dye and another indication for Xalkori, but delayed a decision on a therapy for esophageal cancer. FDA has also accepted a BLA for advanced HER2 breast cancer therapy. Finally, Can-Fite BioPharma plans submission for psoriasis therapy.

Some insulins, as well as some drugs used in emergency care, will now be offered at $0 copay for eligible patients.

A novel therapy in early development aims to permanently turn off the PCSK9 gene in the liver and lower cholesterol with a one-time treatment. It is being developed for a genetic form of high cholesterol.

Drugs approved with an FDA breakthrough designation can provide value that offsets their higher costs, finds study conducted by Tufts Center for the Evaluation of Value and Risk in Health.

In 2023, a wave of biosimilar launches is expected, including seven biosimilars of Humira.

In the future, payers plan to leverage reinsurance and value- or outcomes-based contracting to provide access to high-cost therapies.
As Congress considers legislation that would cap out-of-pocket costs for insulin, Walmart and Civica Rx are taking steps that could make less expensive versions of the diabetes medication more available.
Those with age-related macular degeneration like the port delivery system for ranibizumab because it requires fewer treatments and there is less discomfort.

The FDA expands label for Krystexxa. Agency has granted priority review for two therapies: lecanemab for Alzheimer’s disease and a novel immunotherapy for follicular lymphoma. The FDA will convene a second advisory committee for ALS therapy. Additionally, Bausch + Lomb submits NDA for dry eye disease therapy.
Blue Cross Blue Shield of North Carolina found that uptake of Trikafta was rapid after approval and total cost of care increased 52% despite reductions in hospitalizations and nonpharmacy costs.

Reproxalap is a potential new therapy for dry eye disease with a novel mechanism of action.
NOV03 (perfluorohexyloctane) is a first-in-class eye drop with a novel mechanism of action. If approved it will be the first to address signs and symptoms of dry eye disease.

Under a new add-on program, plan members will have access to an expanded list of FDA-approved specialty generic medications with no copays.
Lecanemab is monoclonal antibody that targets beta amyloid to treat mild cognitive impairment. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) action date of Jan. 6, 2023.