
Kali Panagos, Pharm.D., of ARMSRx discussed ways that pharmacy benefit managers are coping with specialty drug spending.

Kali Panagos, Pharm.D., of ARMSRx discussed ways that pharmacy benefit managers are coping with specialty drug spending.

Kali Panagos, Pharm.D., at ARMSRx, discusses the growing trend of copay assistance programs and alternative funding solutions that can help provide access to expensive specialty therapies.

Doug Long, vice president, industry relations IQVIA, talk about the growth of the specialty medication market and the impact of biosimilars that are expected to be introduced next year.

In COVID-19 news, the FDA indicated Eiger’s treatment not supported by data. An advisory committee switched its vote to yes for new ALS drug. The FDA approved Stimufend, a biosimilar to Neulasta, as well as Imfinzi for biliary tract cancer, a longer-lasting treatment for frown lines, an oral suspension for gastric ulcers, and generics of Revlimid. The agency accepted applications for novel dry eye disease and alopecia therapies. Additionally, Seres completed rolling BLA for microbiome therapeutic.

Because of a generic is now available, Toviaz has been removed and up-tiered on Prime’s Medicare formularies.
Although Xeljanz, Olumiant and Rinvoq are in the same drug class, the risk of serious adverse events from these three products to treat rheumatoid arthritis and other inflammatory conditions may not be similar.

In a second committee meeting, FDA advisors supported approval of AMX0035 after the company presented additional analysis of phase 2 data of AMX0035 to treat patients with ALS. The Prescription Drug User Fee Act (PDUFA) target action date is Sept. 29, 2022.
Prime Therapeutics’ predictive model was able to identify members with high breast cancer pharmacy and medical claims to help clients better manage drug spend.
If approved, NOV03 would be the first prescription eye drop to address excessive tear evaporation. The FDA has assigned a PDUFA action date of June 28, 2023.

A recent literature reviewed showed a plausible link between diabetic retinopathy and the gut microbiome. The authors speculate that the manipulating the makeup of microbiome might be a way to help keep diabetic retinopathy in check.

In COVID-19 news, the FDA authorized updated boosters. The agency also approved several new therapies, including Xenpozyme for a rare genetic disorder, and Pemazyre for myeloid/lymphoid neoplasms, Spevigo for rare type of psoriasis flare. The FDA also expanded Imbruvica’s indication for young children with GVHD and granted priority review for weekly hemophilia A therapy.

Even small payments can influence ophthalmologists and optometrists to prescribe branded therapies to treat patients with glaucoma.
PSG’s latest Artemetrx specialty spend report finds that, as with 2020, claim utilization continues to drive the trend.

Patients who received the chemotherapies paclitaxel and docetaxel were at an increased risk of adverse events of the eye.

Survey participants said their drug costs have gone up and almost a third had trouble paying for food and housing because of high drug costs.

In COVID-19 news, both Pfizer and Moderna have submitted applications for an updated booster vaccine, and the FDA grants EUA for Novavax vaccine for adolescents. The agency also approved rapid-acting Auvelity for major depression, expanded Imbruvica for young children with GVHD, expanded use of Omnipod 5 for younger children, and accepted an NDA for GSK’s myelofibrosis drug.

The PBM is removing four products and adding one product back to its standard control formulary.
Out-of-pocket costs decreased for those with insurance but rose for those without insurance, finds RAND study.

In COVID-19 news, Novaxax seeks EUA for vaccine booster. The FDA has approved Bluebird’s $2.8 million gene therapy for blood disease, as well a high-concentration of Hadlima, a Humira biosimilar. The agency has also PDUFA for several products, including Lynparza/abiraterone prostate cancer, Polivy for a blood cancer, and fezolinetant for menopause.

After a failed trial in advanced breast cancer, Sanofi has stopped all studies of amcenestrant, including in early-stage breast cancer.

Bluebird bio has set a wholesale acquisition cost of $2.8 million for the gene therapy beti-cel, now with the brand name Zynteglo, and is offering an outcomes-based contract with an 80% risk sharing.

Shabbir J. Imber Safdar, executive director, Partnership for Safe Medicines, talks about efforts to address counterfeit drugs in the U.S. supply chain.
Genentech has updated the warnings section of the label for the MS therapy Ocrevus to include information about cases of immune-mediated colitis and a serious brain infection in the post-marketing setting.
Those who spoke at a recent webinar discussed the prescription drug elements of the Inflation Reduction Act, which recently passed in the House, and the impact it will have on Medicare and its beneficiaries.

In a busy week, the FDA has approved additional indications for Enhertu, Myfembree and Nubeqa, as well as a tablet form of Calquence. The agency also approved Xofluza for children, issued an EUA for monkeypox vaccine in children, extended the review of omaveloxolone, set up advisory committee meeting for microbiota-based C. diff therapy, and priority review for elacestrant in breast cancer. Additionally, Novaliq submited NDA for dry eye treatment.

Susan Lang, CEO and founder of XIL Health, talks about the shifting trends and pressures that have led to a misalignment between the PBMs, their clients and consumers.
The Inflation Reduction Act also allows Medicare to negotiate prices on some drugs and caps out-of-pocket costs for Medicare beneficiaries.

A meta-analysis has found that depression and anxiety are significantly correlated with dry eye disease symptoms but not to dry eye disease signs.

The FDA has made several approvals this week, including Enhertu for HER2-low breast cancer, a steroid-free cream for plaque psoriasis and the first interchangeable biosimilar to Lucentis, as well as Stelara in children with psoriatic arthritis. The agency has issued a complete response letter for Nuplazid in Alzheimer’s psychosis. Regulators have accepted a BLA for a biosimilar of Actemra and scheduled a second advisory committee meeting for AMX0035 in ALS.
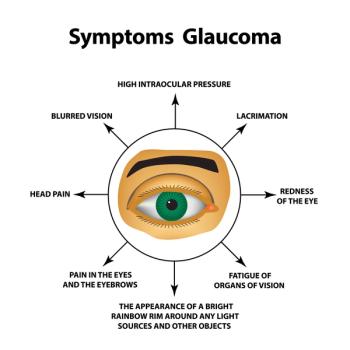
A literature review finds that glaucoma care changed for the better during the COVID-19 pandemic.