Articles by Denise Myshko

VISIBLE is focused on answering data gaps in people of color with psoriasis. Lead investigator Andrew F. Alexis, M.D., hopes the study will generate data to help address care gaps and inform future best practices in diversity research in dermatology.
Checkpoint inhibitors, such as Keytruda (pembrolizumab) and Opdivo (nivolumab), are playing a major role in cancer treatment. But they also produce side effects that affect the skin. Steven Chen, M.D., M.P.H., M.H.P. Ed., said dermatologists need to work with oncologists to manage the side effects so patients can stay on checkpoint inhibitors.

Longer-term use of Opzelura was well tolerated, with no serious treatment-related adverse events, according to a poster presented at the annual dermatology meeting.

Women who are pregnant don’t have to stop all of their treatments during pregnancy. Some can be safely treated for their psoriasis or eczema, according to a presentation today at the annual meeting of the American Academy of Dermatology.

Research shows that short-term exposure to wildfire air pollution can affect the skin and cause flares of certain skin conditions.
After initial treatment, Opzelura used on an as-needed basis was able to control symptoms of atopic dermatitis and helped patients sleep, according to a poster presented at the annual dermatology meeting.
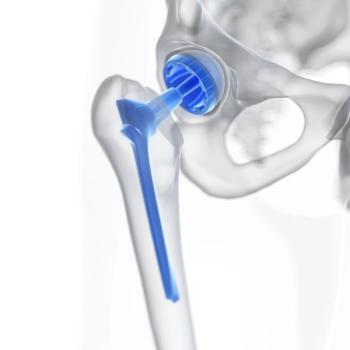
Although not a common complication of hip, knee and other metal implants, the number of allergic reactions is growing, partly the number of joint replacements is growing, according to a presentation at the annual meeting of the American Academy of Dermatology. Nickel is the most common contact allergen.
The VA’s guidelines would exclude anyone who has had a stroke or seizure within the last year, as well as those who have MRIs that show evidence of microhemmorrhages, aneurysms, lesions or tumors.

The FDA has approved a novel nasal spray for migraine and a generic leukemia treatment. The agency has granted priority review to Leqembi for traditional approval but extended the review of hemophilia A gene therapy. Regulators have also assigned target dates for Voxzogo in young children with dwarfism, a skin infection gel, a nerve disorder drug and for Jardiance in children 10 to 17 with diabetes.

Payers are looking to pharmaceutical companies for innovative partnerships that provide patient access, positive clinical outcomes and reduced costs in specialty disease areas, AmerisourceBergen finds.

The FDA has approved Kevzara for an inflammatory rheumatic disease and Skyclarys, the first therapy for rare neuromuscular disease. The agency set an action date for Opdivo for melanoma indication. An advisory committee has supported Pfizer’s RSV vaccine, and Janssen has submitted an NDA for combo tablet for metastatic prostate cancer.

Leqembi would need a 66% to 19% discount from its wholesale acquisition cost of $26,500 a year to fall within commonly used cost-effectiveness thresholds.

The price cuts apply to Lilly’s nonbranded insulin, Humalog, Humulin and its Lantus biosimilar.

Investigators found an association between ICER’s value assessments and how U.S. commercial health plans cover specialty drugs.

The FDA approved several new therapies this week, including a weekly hemophilia A treatment, an extended-release version of Austedo, and the first drug for geographic atrophy due to AMD, as well as an accelerated approval to Filspari for rare kidney disease. The agency also indicated it will hold an advisory committee meeting on Onpattro for heart failure indication, granted priority review for Pfizer’s RSV vaccine and for a life-threatening hereditary immune disease, and also set goal date for blurry vision therapy.

Bruce Feinberg, D.O., of Cardinal Health, said significantly changes are on the horizon for biosimilars, but physician comfort with and use of biosimilars will depend on things beyond their control, including formulary placement and utilization management efforts.

CMS said it will cover monoclonal antibodies to treat Alzheimer’s disease if they are approved through the standard review process and not through an accelerated approval.
Disparities in access to pediatric eye care have increased over the past 15 years and are associated with lower socioeconomic status.

Patients who have had liver problems in the past may be at risk of liver damage from Sprycel, which is used to treat patients with chronic myeloid leukemia.

Starting with Avastin to treat patients with diabetic macular edema — and switching to Eylea if patients don’t have a response — could result in savings of more than $125 million.

Apellis’ Syfovre will have a list price of $2,190 per vial, and Medicare is expected to be the dominant payer.

Sedentary behavior has been linked with low-grade systemic inflammation, which could disrupt the ocular surface.

Navitus will be accessing the Humira biosimilar Amjevita through the lower wholesale acquisition cost option.
Senators want more insight into PBM practices and the role they play in the healthcare ecosystem.

Abarca's Nicole (Gupta) Bulochnik, Pharm.D., talks about how personalized medicine could be integrated into value-based care to accelerate uptake.

Javier Gonzalez, Pharm.D., at Abarca Health, talks about how value-based pricing arrangements can align incentives among stakeholders for specialty disease management.

The branded therapies were removed in favor of generics effective April 2023. CVS Caremark also added 13 generics to its Performance Drug List.

The hearing will discuss the role of PBMs in healthcare and the Pharmacy Benefit Manager Transparency Act, legislation that has been submitted by Sens. Maria Cantwell and Chuck Grassley.

The FDA has approved two pediatric indications this week: Takhyzro for hereditary angioedema and Eylea for retinopathy of prematurity. The agency expanded the label of Cibinqo for atopic dermatitis in adolescents, and granted full approval to Jemperli for endometrial cancer. Additionally, the agency has set PDUFA dates for two drugs: reproxalap for dry eye and zuranolone for depression.
High-priced gene therapies challenge payers to provide access. They are watching the products to be approved the first half of this year.


























