
In total, Medicare beneficiaries paid $21 billion for 100 drugs that receive the most rebates, while health plans paid $5.3 billion after rebates.

In total, Medicare beneficiaries paid $21 billion for 100 drugs that receive the most rebates, while health plans paid $5.3 billion after rebates.

This House bill combines aspects of previously submitted bills — specifically on transparency related to prices on hospital services, laboratory tests, healthcare coverage and PBMs — into one package.

This House bill combines aspects of previously submitted bills — specifically on transparency related to prices on hospital services, laboratory tests, healthcare coverage and PBMs — into one package.
There is a huge opportunity for savings from the recently launched Humira biosimilars and others expected to reach the market in the next few years. But plans and PBMs will need to step into the role that pharmaceutical manufacturers once did in supporting patients and prescribers.

Payers are trying to figure out how to provide access to patients who would benefit from weight loss drugs such as semaglutide but also control costs.

The decision by Scripius (previously Select Health Prescriptions) was made based on the cost of Dupixent, as well as because of an increased demand to use Dupixent for mild atopic dermatitis when the primary patient population is those with moderate to severe disease.

As cell- and gene-based therapies become available for new and expanded indications with a higher incidence of patients, payers will be challenged to find ways to provide access to patients.

PBM transparency and data reporting requirements are likely to be part of a larger healthcare bill that Congress will work on this fall.

The FDA has approved Reblozyl as first-line treatment in MDS-related anemia and the first generics of ADHD drug Vyvanse. The agency also issued a CRL for bevacizumab to treat wet AMD. Additionally, two companies have submitted supplemental applications. These include Janssen, which is seeking full approval for Balversa for urothelial carcinoma, and AbbVie, which is seeking approval of Skyrizi for ulcerative colitis.

Magellan Rx Management has begun providing a multi-state solution in which they negotiate with drug manufacturers for value-based contracts for high-cost gene and cell therapies.

Magellan Rx Management has begun providing a multi-state solution in which they negotiate with drug manufacturers for value-based contracts for high-cost gene and cell therapies.

Researchers at Mass Eye and Ear and Dana-Farber Cancer Institute have developed a new technique for growing stem cells harvested from the eye that meets the FDA’s regulatory requirements for tissue engineering.

The anti-cancer drug bevacizumab is used off-label to treat wet AMD. Outlook Therapeutics had developed an ophthalmic formulation of bevacizumab for intravitreal injection.
Nonprescription Narcan will be available beginning in September in pharmacies and grocery stores, as well as online retailers and have a suggested cost of $44.99.
Experts expect more lawsuits challenging CMS’s authority to negotiate drug prices for Medicare Part D.

Topping the list is Eliquis, which accounted for $16.48 billion in spending for the 3.7 million people in Medicare Part D who take the blood thinner.

Using AI-powered population health analytics, EmpiRx’s model aims to drive better health and financial outcomes with clinical-based pharmacy care.

The FDA has approved the first biosimilar of MS drug Tysabri and granted additional approvals to Abrysvo to prevent RSV in infants, to a higher dose Eylea, and to Ingrezza for a disorder associated with Huntington’s disease. The agency has also set a review date for first-in-class therapy for MDS and granted priority review to Xtandi for earlier treatment in prostate cancer.

Cigna Healthcare has removed prior authorization requirements for a wide range of tests, treatments and medical equipment, but not prescription drugs.
Both commercial and Medicaid plans have added Hadlima to their formularies. Hadlima in both high- and low-concentrations has a price that is 85% off Humira.
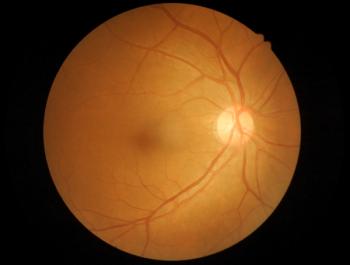
Optical coherence tomography was found to be comparable with MRI in detecting changes to the retina in patients with multiples sclerosis and is non-invasive, inexpensive, fast, and easy to perform.

Carina Dolan, Pharm.D., talks about the trends that are driving both the acute and non-acute spending in hospitals for the next year.

It was a busy week for the FDA. Regulators approved several new therapies, including the first for a rare bone disorder, a combination product for metastatic prostate cancer and a long-acting botulinum toxin for cervical dystonia, as well as an accelerated approved for multiple myeloma. The agency also set review dates for three products, including for full approval of Tarpeyo for a rare kidney disease, an additional indication for Tibsovo for myelodysplastic syndromes and a combination antibiotic for complicated UTIs.

Blue Shield’s new program will unbundle pharmacy services for increased transparency with the goal of saving $500 million annually. The health plan is also teaming up Mark Cuban Cost Plus Drug Company and Amazon Pharmacy.

Blue Shield is replacing CVS Caremark with Prime Therapeutics as the company that will negotiate with drug manufacturers beginning in January 2025. The insurer is also teaming up with Mark Cuban Cost Plus Drug Company and Amazon Pharmacy.

Sohonos was approved to treat children with fibrodysplasia ossificans progressive, an ultra-rare disease that transforms the body’s soft tissue into bone. It has an annual list price of $624,000.

LucyRx has $500 million in equity it will use for acquisitions and to build technology and infrastructure. It aims to be a national, full-service alternative to the big three PBMs.

Kaiser Permanente has a 95% formulary compliance rate, and this allows the health plan to negotiate with pharmaceutical companies for lower prices.

The FDA has approved Talvey, a new therapy for treating multiple myeloma patients. The agency has also issued a complete response letter for avasopasem for severe oral mucositis and set a PDUFA date for a monthly MS drug.
Payers’ requirements for the use of Camyzos in patients with obstructive hypertrophic cardiomyopathy are generally consistent with the registration study, but they also imposed restrictions beyond the FDA indication or ICER recommendation.