
Shortages are growing because of disruption caused natural disasters and ingredient supply issues, as well as increased demand for certain drugs.

Shortages are growing because of disruption caused natural disasters and ingredient supply issues, as well as increased demand for certain drugs.

A new survey finds that more people are using semaglutide and other GLP-1 therapies to lose less than 15 pounds.

FDA has approved a new therapy for HR positive breast cancer and announces plan to hold advisory meeting for Abecma in earlier treatment of multiple myeloma. In addition, Janssen has submitted a supplemental application for Rybrevant in NSCLC.

A new gene therapy administered directly to the brain aims to preserve dopamine neurotransmission in patients with multiple system atrophy.

One notable change: CVS Caremark has removed the Humira biosimilar Amjevita and now prefers Hyrimoz and an unbranded biosimilar.

New biosimilars have been added and others removed from CVS Caremark’s standard formulary. One notable change is with the Humira biosimilars. The PBM has removed Amjevita and now prefers Hyrimoz and an unbranded biosimilar.

Beovu led to greater improvement in retinal thickness and reduction of retinal fluid in patients with diabetic macular edema, a secondary endpoint in this head-to-head trial.

This is the latest offering by Express Scripts that aims to bring transparency to prescription drug costs.

This is the latest offering by Express Scripts that aims to bring transparency to prescription drug costs.
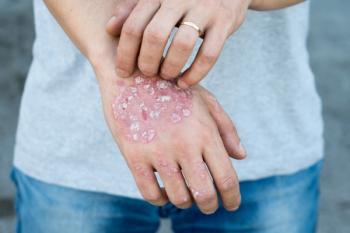
Bimzelx is the first dual IL-17 A/F inhibitor to treat moderate-to-severe plaque psoriasis. It launches with a list price of $7,200 per syringe.

Bimzelx is the first dual IL-17 A/F inhibitor to treat moderate-to-severe plaque psoriasis. It launches with a list price of $7,200 per syringe.

The results of a recent feasibility study on the outpatient administration of cell therapies is creating growing interest in whether home-based management may be possible in the future.

First in class, biosimilars, oncology drugs and even some generics have been added to Optum Rx’s list of exclusions for 2024.

A post-hoc analysis of the SCORED clinical trial identified early benefit for patients taking Inpefa in both heart failure and major adverse cardiovascular events (MACE) related outcomes
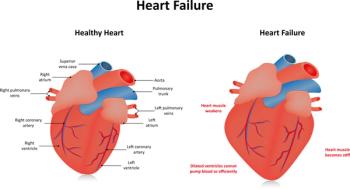
An analysis estimated the clinical and economic impact of Inpefa using inputs from a phase 3 trial of the heart failure drug.

In a phase 2 trial, abelacimab achieved a 99% inhibition of Factor XI and a 67% reduction in major or clinically relevant non-major bleeding events.

A study with a unique trial design and a newer endpoint shows Farxiga can improve cardiometabolic outcomes in a subset of patients.

Beginning Jan. 1, 2024, Cigna Healthcare’s formulary changes mostly impact generics and biosimilars.

In her review of the specialty drug pipeline, Evernorth's Aimee Tharaldson said upcoming approvals for Crohn’s and colitis drugs could further shift the drug spend from the medical benefit to the pharmacy one.
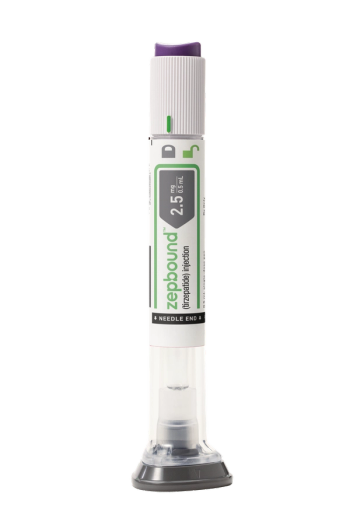
Tirzepatide, with the brand name Zepbound, is expected to be available by the end of the year in six doses at a list price of $1,059.87, which is about 20% lower than semaglutide.

Tirzepatide, with the brand name Zepbound, is expected to be available by the end of the year in six doses at a list price of $1,059.87, which is about 20% lower than semaglutide.

Zurzuvae, the first oral therapy for postpartum depression, will launch in December.

Using an AI platform, Magellan Health was able to better support providers in prescribing behavioral health medications and addressing medication problems, which reduced pharmacy costs and increased adherence.
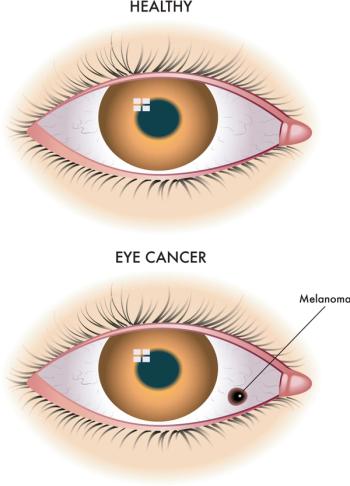
Precision medicine will lead to fewer cases of ocular cancers, earlier diagnosis, and more targeted and sight-saving treatments.

First in class, biosimilars, oncology drugs and even some generics have been added to Optum Rx’s list of exclusions for 2024.

In real-world experience, Tepezza benefits patients with moderate-to-service thyroid eye disease, but adverse events can lead to discontinuation.
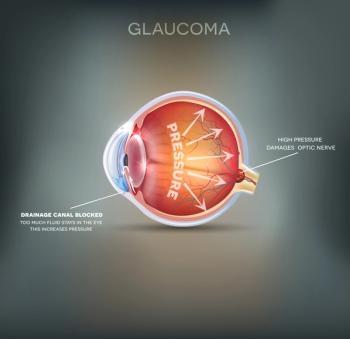
Racial disparities in glaucoma care persist, regardless of socioeconomic status, finds new research presented at the annual meeting of the American Academy of Ophthalmology.

In a late breaking session, a one-time gene therapy was found in a phase 2 trial to provide sustained release of dexamethasone in patients with diabetic macular edema and retinal vein occlusion. The trial is ongoing.
Innovations have allowed retinal procedures to be completed more rapidly with better patient outcomes. One speaker at AAO argues, however, that reimbursement should also consider the complexity of the procedure and not just the time it to takes to complete.

Researchers caution that popular chatbots, while having the potential to improve access and quality of patient education, provide unreliable medical advice.