
Medicare Part B beneficiaries may save between $1 and $3,575 per average dose for the 41 drugs whose prices have risen higher than inflation.

Medicare Part B beneficiaries may save between $1 and $3,575 per average dose for the 41 drugs whose prices have risen higher than inflation.

Medicare Part B beneficiaries may save between $1 and $3,575 per average dose for the 41 drugs whose prices have risen higher than inflation.

A new study finds that the use of continuous glucose monitoring helps reduce complications from type 1 diabetes, lowering the risk of developing or progressing diabetic retinopathy.

New approvals this week include a $4.25 million gene therapy, a drug that treats hypertension in a new way, a nonsteroidal drug for Duchenne, and an oral drug for aggressive leukemia. The agency has set an action date for resubmitted Lymphir in rare skin cancer, and Celltrion has launched Zymfentra, a subcutaneous form of infliximab.

Medicare and Medicaid will cover Wegovy for its recently approved indication to reduce the risk of cardiovascular death, heart attack and stroke in adults with cardiovascular disease and obesity.

This follows AstraZeneca’s and Boehringer Ingelheim’s announcements to also cap their asthma chronic obstructive pulmonary disease drugs at $35.

Tryvio, an oral drug for those with uncontrolled hypertension, will be available in the second half of 2024. The list price has not yet to be determined.

Zymfentra, with a price of $6,181.08 for two shots over four weeks, is approved as maintenance therapy for adults with ulcerative colitis and Crohn’s disease.

Lenmeldy is the first approved gene therapy to treat children with juvenile metachromatic leukodystrophy, a life-threatening inherited disease of the body’s metabolic system.

Lenmeldy is the first approved gene therapy to treat children with juvenile metachromatic leukodystrophy, a life-threatening inherited disease of the body’s metabolic system.
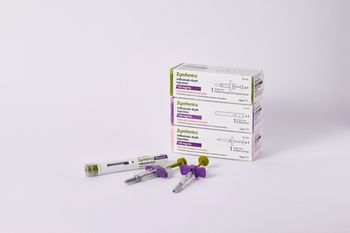
Zymfentra, with a price of $6,181.08 for two shots over four weeks, is approved as maintenance therapy for adults with ulcerative colitis and Crohn’s disease.

The accelerated approval of Iclusig for patients with acute lymphoblastic leukemia and with the Philadelphia chromosome was based on a surrogate endpoint. Iclusig has a list price of $20,831 for 30 tablets.
The accelerated approval of Iclusig for patients with acute lymphoblastic leukemia and with the Philadelphia chromosome was based on a surrogate endpoint. Iclusig has a list price of $20,831 for 30 tablets.

In a recent survey, payers said the Inflation Reduction Act will help lower patients’ out-of-pocket costs, but they were concerned about Medicare’s drug price negotiation and the IRA’s impact on formulary management.

The FDA this week approved a few firsts: the first treatment for NASH and the first CAR-T cell therapy for CLL/SLL. Other approvals include Tevimbra for esophageal cancer; Livmarli for second liver disease indication; and Praluent for children with genetic form of high cholesterol. The FDA also issued a CRL for monthly MS drug. Additionally, Celltrion submitted an application for an interchangeable Xolair biosimilar.
The AI system correctly identified all of the severe cases and accurately detected 80% of the cases with more-than-mild retinopathy of prematurity in a real-world population.

The new collaboration gives Prime access to Capital Rx’s claims system; Prime has become a minority investor in Capital Rx.
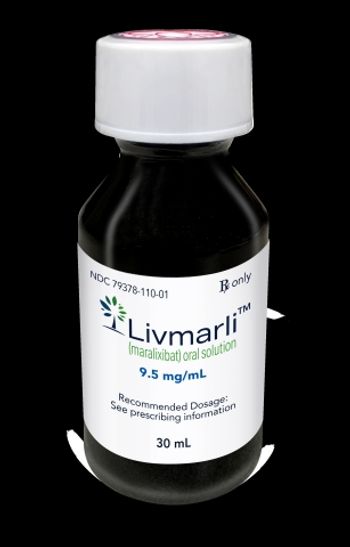
Livmarli is now approved to treat the itching associated two different rare liver diseases: progressive familial intrahepatic cholestasis and Alagille syndrome.

The new collaboration gives Prime access to Capital Rx’s secure claims system JUDI, which Capital Rx leaders say was developed with enhanced security protocols.

Wellpoint is the latest to expand mental health services, teaming up with InStride to offer virtual care for children and adolescents with anxiety and obsessive-compulsive order.
Michigan’s state Medicaid agency has signed an outcomes-based agreement for Lyfgenia patients with sickle cell disease. The agreement provides payers with risk sharing tied to vaso-occlusive events-related hospitalizations

A phase 2 study demonstrated positive results for remibrutinib, a BTK inhibitor, as a treatment for patients with the rare skin condition, hidradenitis suppurativa, finds a study presented at the American Academy of Dermatology Association.
People of color are more likely to develop atopic dermatitis and have more severe disease. Vtama, currently approved to treat plaque psoriasis, is being studied to treat patients with atopic dermatitis.

The FDA has approved Wegovy for reducing cardiovascular risks in people who are obese or overweights. The agency approved new biosimilars that reference Actema, Prolia and Xgeva. Additionally, the FDA plans to hold advisory committee meeting for donanemab in Alzheimer’s disease.
Physicians switch to Opzelura (ruxolitinib) when other therapies fail to help patients with atopic dermatitis, according to a new analysis presented at the annual meeting of the American Academy of Dermatology.

There was no mention in President Joseph Biden's Statue of the Union Address about PBM reform, which has garnered a lot of interest from patient advocates and Congress over the last year.
Regulators want to discuss safety issues, as well as the phase 3 trial’s design where patients were treated based on an assessment of amyloid plaque and the inclusion of patients based on tau protein levels.

The new biosimilar, Tyenne, was approved in both an IV and a subcutaneous forms to treat inflammatory conditions such as arthritis.

Quantile Health is offering a new solution to help small health plans and self-insured employers fund gene therapy access — with a subscription model.
The FLOW trial was stopped early based on an interim analysis that found the study met the criteria for efficacy.