The 3Axis Advisors analysis suggests PBMs’ spread pricing practices leads to employers being charged different amounts for the same medications and to pharmacists facing reimbursement challenges.

The 3Axis Advisors analysis suggests PBMs’ spread pricing practices leads to employers being charged different amounts for the same medications and to pharmacists facing reimbursement challenges.

The newspaper’s investigation, which included interviewing 300 peole, found that pharmacy benefit manager often act in their own financial interest by overcharging employers and government programs.

Nathan Downhour, Pharm.D., discusses the Pharmacy Match program, which will engage with physicians to make sure a specialty prescription gets to the best-fit pharmacy across a network powered by Free Market Health.

Full approval was granted for the one-time gene therapy to ambulatory patients aged 4 years older. The FDA also granted accelerated approval to Elevidys for non-ambulatory patients.

Global mortality fell 13 times faster in countries with a high sociodemographic index compared with countries with a low-middle sociodemographic index.

Physicians in a new survey by the American Medical Association said prior authorization leads to delayed care and a high administration burden for physician practices.
A study in mice, researchers at the University of Michigan have found infection with RSV can alter lung function in young children.

CNN political commentators Scott Jennings and Van Jones briefly touched on the upcoming election’s impact on healthcare at the annual AHIP meeting in Las Vegas.

Hackers are learning health plans’ infrastructure and are attacking the places with the highest value information. Transferring data is the weakest point in the healthcare ecosystem, panelists said at the AHIP meeting in Las Vegas.

Increased health demand, decreased physician workforce, technology advancements and more government involvement in healthcare present challenges — and opportunities — for health plans, said session panelists at the annual AHIP meeting in Las Vegas.

Working with providers to align goals and objectives will lead to better and more successful programs to help manage complex and costly medical conditions, said panelists at the annual AHIP meeting in Las Vegas.

In an interview, Timothy Law, DO, MBA, chief medical officer of Highmark, talks about how GLP-1 agonist drugs like Wegovy and Zepbound are changing the paradigm for weight loss.

In an interview, Timothy Law, DO, MBA, chief medical officer of Highmark, talks about the issues insurers consider when providing coverage for GLP-1 drugs like Wegovy and Zepbound that can help patients lose weight.

Geeta Nayyar, M.D., a rheumatologist and author, discusses how misinformation and disinformation is eroding the trust between doctors and patients, and how healthcare organizations can be part of the solution.

Committee members, however, also said more data are needed on donanemab to treat patients in underrepresented patient groups, including Latin American and African American patients and special populations such as Down syndrome.

Included in this review will be 11 drugs that ICER assessed for cost-effectiveness in 2022. New this year is an assessment for consumer accessibility of drugs, including the burdens of prior authorization and patient cost-sharing measures.

Eating disorders among patients with type 1 diabetes have increased, which increases the risk for serious complications. Patients who skip insulin doses are at a three times greater risk of premature death.

Patients with PTSD in the clinical trials of midomafetamine were able to guess whether they received treatment or placebo. Regulators and advisory committee members said this could have impacted efficacy results. FDA’s decision is expected by Aug. 11, 2024.

A simulation model projects there could savings if patients are switched to lower cost drugs or programs after initial weight loss using GLP-1 drugs.
In a study in mice, researchers found that the COVID-19 virus can infect the retina even when the virus doesn’t enter through the eyes.

The FDA has made two approvals this week: the first interchangeable biosimilar of Soliris and another indication for Breyanzi for mantle cell lymphoma. The agency has delay a decision on Dupixent in COPD and has set goal dates for zolbetuximab in gastric cancer and zanidatamab in bile duct cancer.

LucyRx will begin providing pharmacy benefit services to self-insured employers, covering about half a million lives, beginning in January 2025.

Onyda XR is a liquid form of clonidine that can be used as monotherapy or as adjunctive therapy for patients 6 years of age and older with ADHD.
Yesafili and Opuviz are approved to treat patients with neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema and diabetic retinopathy.

In the United States, spending on oncology therapies rose to $99 billion in 2023, and is expected to grow to nearly $180 billion in 2028, finds a recent report by IQVIA Institute for Human Data Science.

A recent IQVIA report found that uptake of biosimilars on the market for at least three years varies from 8% for insulin lispro to 82% for bevacizumab.
Patients with pulmonary arterial hypertension with commercial insurance lose time from work to seek treatment.

Speakers at the annual American Thoracic Society meeting discussed research looking at whether orangutan respiratory disease syndrome might have some of the same genetic causes as cystic fibrosis.
Patients with more advanced pulmonary arterial hypertension incur higher costs overall, according to a poster at the annual ATS meeting.
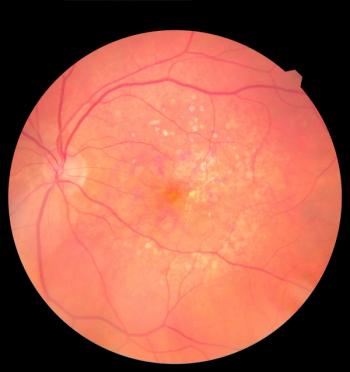
Yesafili and Opuviz are approved to treat patients with neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema and diabetic retinopathy.