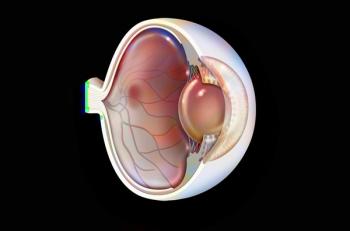
Animal studies of a new compound, which can be delivered by eye drop, have found that it can reverse epigenetic changes that lead to wet age-related macular degeneration.

Animal studies of a new compound, which can be delivered by eye drop, have found that it can reverse epigenetic changes that lead to wet age-related macular degeneration.

ICER has weighed in on two of the 10 drugs selected for negotiations: Eliquis and Xarelto, which are among Medicare Part D’s highest spending drugs.

ICER has weighed in on two of the 10 drugs selected for negotiations: Eliquis and Xarelto, which are among Medicare Part D’s highest spending drugs.

Even though the Inflation Reduction Act will require plans to justify formulary placement of negotiated drugs, payers have more of an incentive to steer patients to lower-cost alternatives.

Once Medicare’s negotiated price for prescription drugs goes into effect, payers have more of an incentive to steer patients to lower-cost alternatives.

Aetna, Cigna, Humana, United Healthcare — some of the largest providers of Medicare Advantage plans — have released updates to prescription drug programs.
The FDA indicated there were no concerns about the safety or labeling of lebrikizumab, which is being reviewed to treat patients with moderate-to-severe atopic dermatitis.

The FDA has approved several new therapies this week, including a new two-component Pompe therapy, an extended-release drug for major depression without sexual side effects, and an eye drop for drug-induced dilation. Additionally, the agency has set review dates for several products, including odronextamab for blood cancers, sotatercept for pulmonary arterial hypertension, and Dupixent in young children with eosinophilic esophagitis.

Exxua is approved to treat adults with major depressive disorder, but its labeling does not contain warnings about sexual function or weight gain.

ICER adds a more formal process to evaluate the diversity of clinical trials and an assessment of a product’s impact on patient and caregiver productivity. ICER also plans to evaluate how newer methods — which would consider the change of a drug’s price over time and disease severity — can be applied to its value assessment.

ICER adds a more formal process to evaluate the diversity of clinical trials and an assessment of a product’s impact on patient and caregiver productivity. ICER also plans to evaluate how newer methods — which would consider the change of a drug’s price over time and disease severity — can be applied to its value assessment.

The FDA has approved GSK’s myelofibrosis drug, as well as a new indication for Jardiance and a new dosing regimen for Talicia. The agency has also issued a complete response letter for epinephrine nasal spray and assigned a review date for the gene therapy atidarsagene autotemcel.

Developing novel drugs to treat cancer remains a strong focus for drug developers. Several first-in-class therapies have become available over the last few months.
An analysis from 3 Axis has found that PBMs control how much pharmacies are reimbursed. This can vary significantly depending on the PBM contracts with insurers, creating a system with large inconsistencies in the prices that consumers pay for both generics and branded products.

An analysis by 3 Axis Advisors has found that there is a large variability in pharmacy reimbursement of prescription drugs depending on PBM contracts with insurers. This creates a system with huge inconsistences on the prices of both generics and branded products.
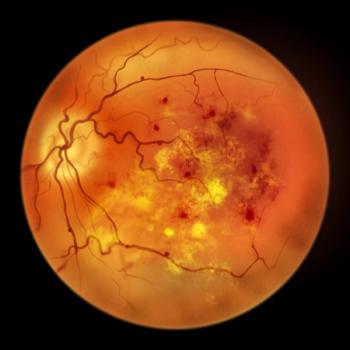
MSU researchers are hopeful that this new insight about cholesterol crystals in the retina could provide an opportunity for earlier diagnosis and treatment of diabetic retinopathy.

The FDA has requested an additional study. ARS Pharmaceuticals plans to appeal the decision.
Even though committee members supported use of Onpattro for patients with cardiomyopathy related to transthyretin-mediated amyloidosis, they had questions about whether it provided a clinically meaningful benefit. The FDA set an action date of Oct. 8, 2023.
A workshop of the International Agency for Research on Cancer looked at the gaps in knowledge of four different cancers associated with HIV: Kaposi sarcoma, cervical, lung and anal cancer.

Most commercial payers cover remote physiologic monitoring, but coverage of electronic consults and electronic visits is less consistent. Commercial plans also have different levels of transparency.

The week, the FDA approved Aphexda for use in stem cell mobilization. The agency also assigned action dates for resmetirom to treat patients with NASH and Libervant Film for young children and extended the review for lifileucel to treat melanoma. Additionally, an FDA advisory committee backed the use of Onpattro in cardiomyopathy of ATTR amyloidosis.
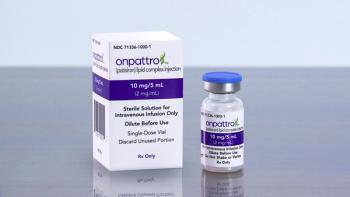
Even though committee members voted in support of Onpattro for patients with cardiomyopathy related to transthyretin-mediated amyloidosis, there were questions about whether it provided a clinically meaningful benefit. The FDA set an action date of Oct. 8, 2023.
CMS has included an additional 34 drugs on the list on products subject to rebates if their price increases are higher than inflation.

Akili executives said a focus on non-prescription digital therapies removes the barriers payers place on access and creates a more sustainable business.

The assessment framework developed by Peterson Health Technology Institute and the Institute for Clinical and Economic Research will prioritize products’ clinical benefits and economic impact of digital health technologies. The companies will announce the first set of health technologies to be reviewed by mid-October.

Cell and gene therapies can be used for patients with cancer and serious diseases who have limited or no treatment options — but at a cost.

Addressing drug affordability and accessibility will involve tackling the interrelated problems of high prices and problematic pharmacy benefit manager practices, says Benjamin N. Rome, M.D., with Brigham and Women’s Hospital in Boston.

The tentative approval by the FDA is through the President’s Emergency Plan for AIDS Relief (PEPFAR) program and is supposed to ease regulatory authority submissions, production and distribution in low- and middle-income countries.
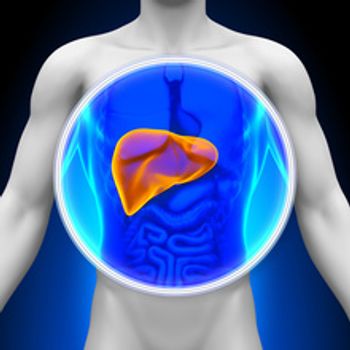
About 65% of adults in the United States drink sugar-sweetened beverages daily, which increases the risk for liver cancer and mortality in women.

In total, Medicare beneficiaries paid $21 billion for 100 drugs that receive the most rebates, while health plans paid $5.3 billion after rebates.