
In some cases, the exclusions favored brand medicines that were more expensive than the excluded generics.

In some cases, the exclusions favored brand medicines that were more expensive than the excluded generics.

This trend in prescription drug pricing outpaces growth in prices for other healthcare services.

The commission will review PBM business practices, including the impact of rebates on formulary design, the costs of prescription drugs to patients, and methods to determine pharmacy reimbursement.

A more accurate number of rare diseases is more than 10,000, and just 500 of these disorders have treatment options available for patients, according to RARE-X.

Xcenda’s review of the three largest PBMs—CVS Caremark, Express Scripts, and OptumRx—finds they are excluding medicines in classes where generics are not available or for serious conditions, such as oncology and autoimmune disorders.

An Xcenda survey of payers found that cost-effectiveness analyses from the Institute for Clinical and Economic Review (ICER) are used in price negotiations and to implement prior authorizations.

The FDA has withdrawn the approval of Ukoniq, which is used to treat two specific types of lymphoma. In approvals, the regulatory agency has approved a new indication for Kymriah, two addition Opdivo regimens, and extended the use of Evrysdi in newborns with SMA. The agency also accepted for review Dupixent to treat skin lesions but extended the review of a new ALS therapy, refused to file an application for a rare metabolic disorder, and put a hold on the trial for Cialis OTC.
The FDA has extended review time to allow for review of additional analyses of data from the company’s clinical studies. The new PDUFA date is Sept. 29, 2022.
The label now includes a new section about the risk of substituting an oral azacitidine product, Onureg, for the injection therapy.

More than 5,700 trials worldwide are investigating PD-1/PD-L1 inhibitors, and new trials of these therapies were of combination regimens.

The FDA indicated the risk of death outweighs the benefits of Ukoniq, which was approved in February 2021 to treat specific lymphomas.

If approved, Orasis’ low-dose pilocarpine would be the second product that improves presbyopia, which is the age-related loss of clear up-close vision. The company plans to submit an NDA in the second half of the year.

The bill would allow the Federal Trade Commission to impose penalties on PBMs of up to $1 million for unfair and deceptive practices.

The FDA has approved the first non-steroid cream for psoriasis, as well as another biosimilar of Neulasta, Tibsovo combination for older patients with leukemia and a new formulation of Tyvaso. The agency has issued a second CRL for poxvirus treatment and also accepted applications for several therapies, including a nasal spray for migraine, mirvetuximab for ovarian cancer, and a treatment for the rare disease Friedreich’s ataxia.

Molly Beinfeld, senior research lead, evidence synthesis at the Institute for Clinical and Economic Review, talks about the organization’s research assessing payer coverage policies of prescription drugs.

Doug Nemecek, M.D., chief medical officer, behavioral health at Evernorth, talks about the mental health issues teenagers are facing.

Launched last year, Prime’s MedDrive is an integrated drug management solution that leverages biosimilars to help reduce drug spend.

In COVID-19 news, FDA expanded EUA for Pfizer/BioNTech COVID-19 booster to children 5 to 11 years and cleared first at-home combo COVID-19, RSV and flu test, but declined an EUA for the antidepressant fluvoxamine to treat COVID-19. Regulators also approved Lilly’s novel diabetes drug and Dupixent eosinophilic esophagitis, modified Dsuvia REMS program and issued a CRL for bimekizumab for psoriasis.

Generics are now available for the two drugs: Vimpat and Combigan.
The FDA has changed how often AcelRx Pharmaceuticals is required to audit healthcare settings that administer Dsuvia, which is a synthetic opioid.

Aetna has made available four specialty drugs that were approved early in 2022 through prior authorization. These include a biosimilar, a CAR-T immunotherapy, and therapies for macular degeneration and a rare auto-immune disease.
While regulatory flexibility is important for drugs for rare diseases, investigators are concerned the trend toward surrogate endpoints decreases confidence that new drugs can improve patient outcomes.

FDA regulators said the data to support the EUA, which was submitted by a physician, is not sufficient to support the use of the antidepressant fluvoxamine for the treatment of patients with COVID-19.

The role of health economic and real-world evidence has become, and will continue to be, an important aspect of healthcare decision-making.
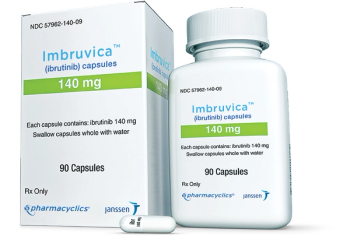
The label for Imbruvica now includes information about the possibility of cardiac failure, which has occurred in 1% of patients. Imbruvica is used to treat B-cell blood cancers.

In COVID-19 news, the FDA has approved Olumiant for new COVID-19 indication, but had limited Janssen’s COVID-19 vaccine. The agency has launched a new program for rare disease drug development and approved a new oral form of ALS therapy. Regulators have also extended the review time for both a new Pompe disease therapy and the sNDA for Myfembree for endometriosis. Additionally, Eisai has completed its submission of lecanemab for Alzheimer’s disease.
Apokyn and Kynmobi contain apomorphine hydrochloride, which can cause hemolytic anemia that requires hospitalization. The products both treat the loss of muscle movement control caused by Parkinson’s disease.
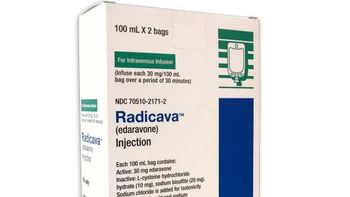
Radicava ORS has the same dosing regimen as the IV form of the ALS therapy, with a treatment cycle followed drug-free periods.
The FDA needs more time to assess information submitted by the company.

Financial hardship was more likely to be reported by older patients who don’t have insurance, take multiple medications, and have a low annual household income.