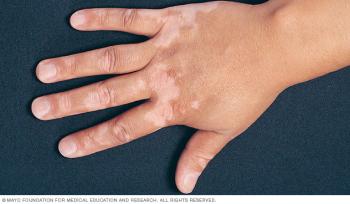
Opzelura is being reviewed as a treatment for vitiligo, a disease that causes the loss of skin color. The new PDUFA date is July 18, 2022.

Opzelura is being reviewed as a treatment for vitiligo, a disease that causes the loss of skin color. The new PDUFA date is July 18, 2022.

A meta-analysis finds that providing both parents and children with asthma with information about managing their disease can reduce emergency room visits and hospitalizations.

The agency plans to convene an advisory committee to review the application of Nuplazid to treat Alzheimer’s psychosis. The FDA also approved an indication for Opdivo, okays a third biosimilar to blockbuster cancer treatment Neupogen, and issues complete response letter for long-acting HIV therapy.

JAK inhibitors, such as Jakafi (ruxolitinib), have helped improve outcomes and survival of patients with the rare blood cancer.

The company will distribute nalmefene for no profit, which is part of the company’s bankruptcy filing and settlement with states.
With a PDUFA date of Aug. 4, 2022, the FDA plans to hold an advisory committee meeting on resubmitted data for Nuplazid in patients with Alzheimer's disease who have psychosis.
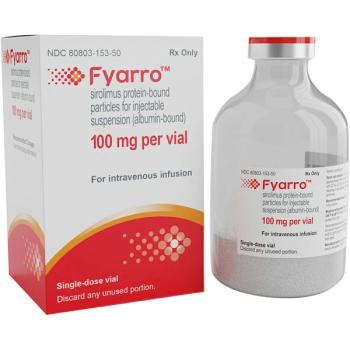
Aadi Bioscience recently launched Fyarro, the first FDA-approved therapy to treat an ultra-rare sarcoma.

Generics are now available for the three therapies: Cystadane, Selzentry, and AmBisome.
The FDA cited issues related to the compatibility of lenacapavir and the vial used. The agency has not requested new clinical studies.

A total of 28 lots have been recalled because of a high rate of allergic reactions.
Over time, Egaten, an antiparasitic, can increase the QTc interval, resulting in a heart rhythm condition that can cause chaotic heartbeats.
Once approved, the insulins will be available to patients for no more than $30 per vial.

2021 was a year in which investigators, government officials and drug companies overcame immense challenges to develop successful vaccines against COVID-19. That spirit of innovation is expected to carry over into this year.

Reporting of PRO in oncology clinical trials increased after standards from ISOQOL and others were published in 2013.

This is the Areva's second importation of bupivacaine this year.
During the period 2015 to 2020, if a proposed policy that increases Medicaid rebates for drugs with accelerated approvals had been enacted, up to $5.2 billion could have been saved.
Because of a COVID-19 related shortage, facilities may not have immediate access to Actemra, which is required as part of the REMS programs for CAR T-cell therapies.

Consumers have a false sense of security about buying drugs from online platforms.
Researchers suggest that adjusting the prescribing patterns of about 8% of physicians could result in substantial savings.

In COVID-19 news, FDA grants EUA to AstraZeneca’s monoclonal antibody to prevent infection and the FDA extends EUA of Pfizer/BioNTech Booster to those 16 and 17 years of age. The regulatory agency also approved a therapy for spasticity resulting from multiple sclerosis, and is requiring an updated boxed warning on three top-selling JAK inhibitors for rheumatoid arthritis. And FDA committee votes no on kidney disease drug.

Medexus is working with Ethypharm to generate the data needed to support an abbreviated new drug application for triamcinolone hexacetonide.
The regulatory agency is reviewing Reata’s application of bardoxolone to treat patients with chronic kidney disease caused by Alport syndrome, a rare genetic disease.
In this diagnostic proof-of-concept trial, an emerging imaging approach that uses a handheld laser demonstrated the ability to visualize muscle degeneration in children with SMA.
Drugs in development and newly approved drugs for non-small cell lung cancer are based on research that has identified driver mutations leading to runaway cell growth and division that characterize cancer.

The pandemic has been associated with an increase in public health spending, rising mental health issues and a record number of overdose deaths, according to a report from the philanthropic arm of UnitedHealth Group.

In COVID-19 news, an advisory committee recommends approval of oral antiviral after Merck releases updated data. The FDA made three approvals this week: an imaging agent to detect ovarian cancer and a vaccine for hepatitis B, and Darzalex Faspro is given an additional indication. New regulatory applications include: Lynparza for early breast cancer; the psoriasis therapy deucravacitinib, a pneumococcal vaccine for children, and an anemia therapy. The regulatory agency also paused the updated clozapine REMS program.
The warnings section of Inrebic, a JAK inhibitor to treat a bone marrow cancer, now includes information about the cardiovascular risk associated with Xeljanz, another JAK inhibitor to treat arthritis.

ICER’s first scorecard of the barriers to fair access to medications found, of the policies that could be reviewed, most were structured to support fair access to medications.

ICER’s first scorecard of the barriers to fair access to medications found that more transparency is needed to fully assess insurers and their coverage policies.

A new meta-analysis of research finds that a history of previous cardiac events puts children with hypertrophic cardiomyopathy at risk for cardiac death.