
Psychiatrists and psychologists also had lower in-network reimbursement rates.

Psychiatrists and psychologists also had lower in-network reimbursement rates.

A group of researchers recently aimed to investigate the prognosis of patients with NSCLC that lacked a spouse, family members, or friends to support them during cancer treatment.

Panelists discussed reaching beyond large patient advocacy groups for input and how clinical trial endpoints may paint an incomplete picture of a drug's true value to patients.

Ross Margulies, J.D., M.P.H., a partner at the Foley Hoag law firm, discusses whether proposed transparency rules in the pharmacy benefit manager (PBM) legislation that Congress is considering might apply to the PBM-affiliated group purchasing organizations.

Ross Margulies, J.D., M.P.H., a partner at the Foley Hoag law firm, discusses proposals to “delink” what pharmacy benefit managers are paid from the price of drugs.
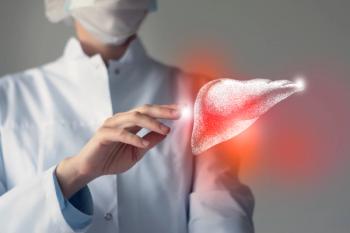
The pipeline for NASH treatments is robust, with six therapies in phase 3 development. Speakers at the annual AMCP meeting discuss Rezdiffra, the first drug approved specifically for NASH, and how to think about coverage issues.

According to the co-author of a poster presented at the annual AMCP meeting hosted in New Orleans this week, the interest in the study where the data was found stemmed from the prevalence of PPD in adults, especially women after giving birth.
Factors preventing biosimilar access include pharmacy benefit manager contracts and pharmacies not communicating with providers, a survey finds.

Ross Margulies, J.D., M.P.H., a partner at the Foley Hoag law firm, says there is broad agreement on transparency provisions and a ban on spread pricing in Medicaid.

The Access to Prescription Digital Therapeutics Act remains a top priority for the Academy of Managed Care Pharmacy (AMCP), according to Jennifer L. Mathieu, AMCP senior vice president of professional and government affairs.

Generation Z (1997–2012). Everything on demand, including healthcare

CEO John Baackes is stepping down at the end of this year.

In a slow week, the FDA has approved Fasenra for children 6 to 11 years of age with eosinophilic asthma, a rare form of asthma.

On specialty drugs, the Prime acquisition and vertical integration

Gen X (1965–1980), still traditional — and stuck in the middle.

The need for tools that effectively route patients to the right care at the right time has intensified. But relying only on a tech-enabled approach heightens the potential for missed human connections.

The proposed rule for the fiscal year (FY) 2025 Inpatient Prospective Payment System and Long-Term Care Hospital Prospective Payment System introduces strategies to improve the well-being of Medicare beneficiaries, including efforts to tackle social determinants of health, create more robust emergency readiness and enhance maternal healthcare.

The average American can afford a maximum of a $97 for out-of-pocket healthcare expenses.

Ten biosimilars to Humira are now on the market, but AbbVie’s Skyrizi and Rinvoq are soaking up revenues as Humira’s sales fall off.

How COVID-19 became a jumping off point for HIV vaccine conspiracies.

If the company' experimental agent is approved, it would join Humira (adalimumab), Rinvoq (upadacitinib) and Skyrizi (risankizumab) in AbbVie's portfolio.

A new study finds that of the patient portal messages that result in a bill, most involved physicians helping patients with high blood pressure or diabetes.

The decision comes after the FDA Oncologic Drugs Advisory Committee provided a unanimous vote recommending approval. Carvykti is now available to treat patients after just one prior line of therapy.

The FDA has approved several new therapies this week, including Voydeya as an add-on therapy for blood disorder PNH; Zevtera for serious bacterial infections; Fanapt for bipolar 1; and the CAR-T therapy Abecma for earlier treatment in multiple myeloma. The agency has also accepted a supplemental BLA for Bimzelx in hidradenitis suppurativa and set a review date for datopotamab deruxtecan in breast cancer.
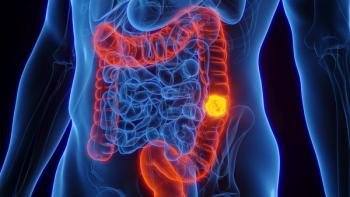
Colorectal cancer is now the leading cause of death in men and the second leading cause of death in women.

The chimeric antigen receptor T-cell (CART-T) therapy is now approved to treat patients with multiple myeloma after two prior lines of therapy.

Darina Georgieva, Pharm.D., and her colleagues from the department of pharmaceutical services at Vanderbilt University Medical Center, conducted a retrospective observational study to learn the costs avoided through specialty pharmacist interventions for patients at the Vanderbilt MS Clinic. The study results were published in the Journal of Managed Care and Specialty Pharmacy earlier this month.

Approved in late September 2022, Relyvrio (sodium phenylbutyrate/taurursodiol) will no longer be available for new amyotrophic lateral sclerosis (ALS) patients as of yesterday.

The efforts of the final rule aim to simplify the enrollment process for low-income consumers, provide states with more flexibility in offering routine adult dental services and set guidelines to create access to in-network providers.

Market shares have fallen for CVS, UnitedHealth Group and more following the announcement.