
Progentos will use the funding to advance its multiple sclerosis (MS) program through human proof of concept clinical studies and expand its small molecule pipeline to other degenerative conditions.

Progentos will use the funding to advance its multiple sclerosis (MS) program through human proof of concept clinical studies and expand its small molecule pipeline to other degenerative conditions.

Fears also extend to the future of Social Security, which may run out by 2036, according to the Social Security Office of Retirement and Disability Policy.

In the latest episode of the Meet the Board podcast series, Managing Editor, Peter Wehrwein, catches up with Chief Clinical Officer at Optum Rx and EAB member, David Calabrese, about his new role alongside his team's progress in improving quality, patient safety and health equity in healthcare.

Three-quarters of disenrollments were due to procedural reasons such as incomplete applications, application errors and inaccurate contact information.

Telehealth companies, Costco, other companies — they are all looking to take advantage of the soaring demand for the weight loss drugs.

A simulation model projects there could savings if patients are switched to lower cost drugs or programs after initial weight loss using GLP-1 drugs.

A growing proportion of small- and medium-sized employers are self-insuring. But with self-insurance, comes fiduciary responsibility and possible scrutiny.
In 2022 alone, an estimated 10.6 million new TB cases were reported, mainly in low-income regions, highlighting the urgent need for further efforts in TB prevention and control.
A SCAN Health Plan "deprescribing" program is working to identify unnecessary prescriptions. In 2023, the program resulted in an estimated $2 million in savings from avoided fractures, falls, visits to the emergency room and other adverse drug events.

Drug developers hope to get in on the action in the hot-selling weight loss drug class

This is the first new recommendation from the American Academy of Pediatrics since the AIDS epidemic began in the 1980s.

The FDA has made two approvals this week: the first interchangeable biosimilar of Soliris and another indication for Breyanzi for mantle cell lymphoma. The agency has delay a decision on Dupixent in COPD and has set goal dates for zolbetuximab in gastric cancer and zanidatamab in bile duct cancer.

COBRA coverage is not currently considered creditable coverage, so some seniors face Part B penalties. That needs to change.

Healthcare providers and community leaders won’t need a green thumb to implement these changes.

More than 25% of low-income families' earnings go toward healthcare costs.
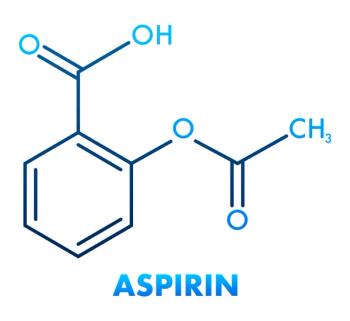
Some large randomized trials have suggested that taking low doses of aspirin on a daily basis would be an easy, inexpensive way of lowering the risk of getting age-related macular degeneration. Not so a well-designed, placebo-controlled study of septuagenarians conducted in Australia.

On the heels of Winrevair's approval, Gossamer Bio and the Chiesi Group struck a deal to develop seralutinib.

Researchers find a mismatch between demographics of the neighborhoods where the FQHCs with vision care are located and those using the vision services.
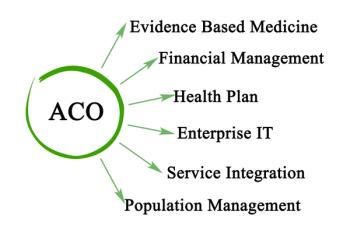
CMS recently introduced a new ACO model designed to ease the cost of setup for low-revenue ACOs focusing on primary care. The ACO Primary Care (PC) Flex Model will launch on New Year’s Day 2025.

Although bills that would make comprehensive changes to the PBM industry have advanced in Congress, Paul Kelly, a veteran healthcare lobbyist, says a limited version that saves Medicare money may pass as an offsetting "pay for" for extension of more liberal rules for telehealth services that Medicare covers.
Wrong diagnoses were also made in women with dense, fatty breasts.

At the 2024 American Thoracic Society conference, Surya Bhatt, MD, director of the Lung Imaging Lab at the University of Alabama of Birmingham discussed the NOTUS trial, a second phase three trial for treating COPD with type two inflammation using dupilumab, following the BOREAS trial.

Veteran healthcare lobbyist discussed the prospects for the Senate passing comprehensive PBM legislation in the most recent episode of Managed Healthcare's DC Roundtable podcast.

MHE Editors are seeking diverse healthcare professionals from different backgrounds and healthcare sectors, with individual interests. Eligible candidates are early or mid-career leaders with less than 10 years of experience. Award winners will enjoy complimentary passes to the PBMI Annual National Meeting in Orlando, Florida, from Sept. 4-6. Additional perks include a feature in our August issue, a subscription to MHE and more!

Preliminary data suggest people with Group 1 pulmonary hypertension had improved outcomes if they had ever received hormone replacement therapy.

An administration change in November could see the return of closed borders or increased protections for immigrants.

Undocumented individuals are ineligible for the Affordable Care Act (ACA) or federally funded Medicaid, but some states offer state-sponsored coverage for certain immigration statuses, such as Temporary Protected Status.

Immigrant children need a variety of resources when they come to the United States.