
American Indian and Alaska Native women also had higher comorbidities and less access to private insurance than White women.

American Indian and Alaska Native women also had higher comorbidities and less access to private insurance than White women.

But the presenter downplayed the rivalry between the two new treatments for geographic atrophy. “I think we are lucky to have multiple therapies,” saidPaul Hahn, M.D., Ph.D. of NJRetina.

How Long COVID-19 rates differ across the pre-delta, delta and omicron eras.

The study's results were presented today at the American Society of Retina Specialists meeting in Stockholm, Sweden, by Parisi Emami-Naeini, M.D., M.P.H., a retina specialist at the Tschannen Eye Institute at the UC Davis Eye Center.
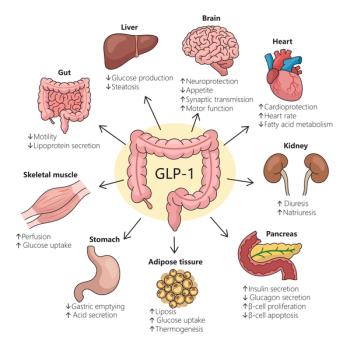
But the Mayo Clinic ophthalmologist who presented the findings stressed that it was “hugely reassuring” the GLP-1s were comparable to older diabetes drugs with respect to retinopathy given all their other advantages.

Dr. Charles C. Wykoff discusses how a one-time gene therapy could benefit patients with wet age-related macular degeneration.

Dr. Charles C. Wykoff discusses interim results from a phase 2 trial of a gene therapy to treat with patients with wet age-related macular degeneration.

UnitedHealth is still feeling the impact of the Change Healthcare cyberattack.
Biosimilars generally enter the market priced at roughly 50% of the amount of their reference drugs.

Geoffrey G. Emerson of Retina Consultants of Minnesota and medical director at the Phillips Eye Institute in Minneapolis and an associate adjunct professor at the University of Minnesota, previews the 2024 annual meeting of the American Society of Retina Specialists (ASRS) starting Wednesday, July 17, in Stockholm, Sweden.

Grandmother and great-grandmother Donna Osborne had recently finished radiation therapy when she won the prize.

Developed by Vertex Pharmaceuticals, suzetrigine is a promising new class of pain medicine that has been gaining attention for its innovative mechanism of action and reported efficacy; suzetrigine might offer hope for those struggling with acute pain issues who want to avoid opioids and NSAIDs.

How AI-powered technology and its data-driven insights improve payer operations and reimburse providers faster.

The retail giant shuttered its primary healthcare clinics, and CVS and Walgreens are cutting back. Is retail healthcare doomed for failure?

Lotilaner ophthalmic solution 0.25% drops are administered as twice daily for six weeks. Other ways of treating Demodex blepharitis manage symptoms, not the root cause, and are of indeterminate length.

: A Taiwanese study builds upon previous contradictory research exploring the link between breast cancer and acute myocardial infarction.

One theme of the Federal Trade Commission’s interim take on pharmacy benefit managers is vertical integration and how that affects competition in the industry.

Community health workers can improve health outcomes and lower costs. So why haven’t they been fully incorporated into the U.S. healthcare system?

Gene therapies are incredibly expensive and tend to benefit small numbers of people, a combination that presents myriad financing challenges for patients, providers, payers and producers. A new study explored some of the options.

Here’s what you missed this week on Managed Healthcare Executive.

Researchers from the University of Utah in Salt Lake City have developed a more comprehensive, multifaceted digital tool for lung cancer screening that integrates into EHR as well as patient portals. Their paper was published in JAMA Network Open in June 2024.

The St. Louis-based center will serve as a base where researchers and public health experts can fight the HIV epidemic together.
The market for biosimilars seems to be improving, partly because of provisions in the Bipartisan Budget Act of 2018 and the Inflation Reduction Act of 2022.

This is good news for developing an HIV vaccine because previous research had indicated it could take years for these antibodies to appear.

The speed of adoption of new practices in medicine is extremely slow. We need systems to test new protocols, make sure they’re safe and effective, and get them out into the world more rapidly.

The FDA may approve KarXT, an antipsychotic that does not have the side effects of some other antipsychotics because it does not affect dopamine receptors.

A breakdown of recent survey results revealing which benefits employers plan to keep and what they are looking to add in 2025.

Previous acupuncture studies have focused on European and American women, but little was known about breast cancer patients in Asia.

Loren McCaghy, director of consulting, health and consumer engagement and product insight at Accenture, shared the results found from his organization's latest report on how many consumers are switching their healthcare providers and insurance payers. In the interview, he touched on some of the main drivers to consumers leaving and solutions on how payer and provider groups can turn things around.

The newspaper’s investigation, which included interviewing 300 peole, found that pharmacy benefit manager often act in their own financial interest by overcharging employers and government programs.