Blood Cancer
Latest News

Latest Videos
CME Content
More News
The outlook for leukemia patients improved after Gleevec (imatinib) was approved in 2001 as a treatment for chronic myeloid leukemia. The American Cancer Society published its annual cancer statistics report today that projects 62,770 new cases of leukemia will be diagnosed this year and 23,670 deaths from the disease will occur.
Results for Amgen’s Blincyto (blinatumomab) suggest it has efficacy as a treatment for Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia.
Researcher modeling the cost effectiveness of chimeric antigen receptor T-cell therapy (CAR-T) therapy and antibody conjugate found that they fell short.

The Covid-19 pandemic revealed the advantages of treatment that involved fewer in-person office visits, according to research presented at American Society of Hematology meeting earlier this month. Researchers also saw a notable increase in the use of Darzalex (daratumumab).

Johnson & Johnson’s Tecvayli (teclistamab-cqyv) has been approved by the FDA, and Pfizer’s elranatamab received breakthrough status that should speed its approval. Bispecific antibodies may be a bridge to CAR-T treatments or an alternative to them.
The younger Black patients with acute myeloid leukemia (AML) had “alarmingly high” early death rates within the first 30 days of study enrollment, indicating possible delays in diagnosis and care, according to research results reported in the journal Blood Advances.
Positive results for Adcetris (brentuximab vedotin) were reported in The New England Journal of Medicine. Further research will include a look at the cost effectiveness of Adcetris.

The FDA approved Tecvayli (teclistamab) yesterday after approving two CAR-T therapies as late-line treatments for multiple myeloma earlier this year. Because it is ‘off the shelf,’ Tecvayli may have advantages over the CAR-T therapies, which are custom made for each patient and involve harvesting the patient's T cells.
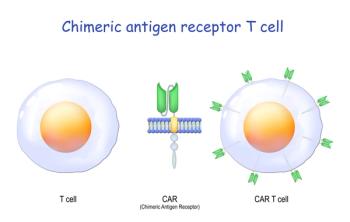
Durability and toxicities such as cytokine release syndrome and immune effector-cell neurotoxicity syndrome are among the challenges.

The study looked at cancer patients as a group, but senior author notes that ‘that treatments for blood cancers are very costly and many of them require treatment for long periods of time.’
For almost all the patients with stage I or stage II disease, the cumulative incidence of CVD mortality exceeded that of classical Hodgkin lymphoma and other cancers, according to research results published in the journal Cancer.

The researchers attributed low adherence to oral anti-cancer medications for blood cancer and certain other cancers to high monthly out-of-pocket among several other reasons.

Researchers report that half of the online pharmacies are rogue operations that may operate without a license or have other serious shortcomings.

MD Anderson’s Patrick Reville says preclinical research shows combining CAR-T therapy with other treatments may make it more effective as treatment for large B cell lymphomas.
The FDA has approved two CAR-T therapies and a review of bispecific antibody is underway.

Results show that the mortality risk was cut in half for patients who got help with the financial burden of their care. Quality of life scores also improved.

An Ibrance-Jakafi combination shows promise in a mouse model of myelofibrosis.
Lack of antibodies from vaccination mainly affects patients with B cell cancers. Experts say antibodies are just one piece of the puzzle and that other aspects of the immune system fend off serious cases of COVID-19.

The confirmatory trial of one of the approved drugs, Pepaxto, was halted when data suggested it was associated with a higher mortality rate.
NCCN adds Rylaze to guidelines soon after was approved by the FDA and a British company reports positive results for its CAR-T therapy.

New research and updated treatment guidelines add to knowledge base on this rare blood cancer.

The Leukemia & Lymphoma Society is providing grants to increase trial enrollment of ethnic and racial minorities.
Researchers are making progress in identifying drugs that target certain mutations.
NCCN recommends Brukinsa for CLL/SLL even though it is FDA-approved for mantel cell lymphoma.

Deposing the ‘Emperor of All Maladies’ will take a diverse toolkit, not a single moonshot.

























