Pharmacy
Latest News

Latest Videos

CME Content
More News

Coverage decisions about prescription digital therapeutics may take payers out of their comfort zones and require new processes and subject matter experts, according to members of a panel at the AMCP annual meeting. But P&T committees are likely to still be central to the process.

They are the trendy way to lose weight and payers have been noticing an increase in GLP-1 claims. An analysis of the pharmacy and healthcare claims of a small commercial health plan in Texas documents the growth in the off-label usage of the GLP-1s, such as Ozempic, for weight loss.

The IQVIA vice president sees trouble for generics manufacturers, but painted a bright picture for biosimilars during his keynote address at the annual meeting of the Academy of Managed Care Pharmacy (AMCP).


The co-anchor of Good Morning America spoke about her experience when she was diagnosed with myelodysplastic syndrome.

Costs have always figured into drug coverage decisions, but value assessments have become a separate and more formalized part of the process at payer organizations, said members of a panel on value frameworks at the 2023 AMCP annual meeting. The Institute for Clinical and Economic Review (ICER) came up often during the discussion.

A pavilion devoted to prescription digital therapeutics and an educational track devoted to biosimilars are new this year.

This portion of the month's cover story series spotlights PBM EmsanaRx, a branch of Purchaser Business Group on Health (PBGH), and its President and CEO, Elizabeth Mitchell, who explains that EmsanaRx differs from other PBMs because they use what PBGH calls a waste-free formulary. This months cover story shines a light on the companies, trends and ideas that are shaking things up and reshaping the contour of how healthcare is paid for and delivered.

Senators and witnesses said at a Commerce Committee hearing today that pharmacy benefit managers are putting independent pharmacies out of business and siphoning money from the drug supply chain. A University of Chicago economist and the industry trade association pushed backed, saying they bargain to save payers and patients money.

The announcement of the $10.6 billion acquisition comes a few months after the company announced it was buying Signify Health, a home health provider, for $8 billion.

In 2022, 12 states have enacted 19 pieces of legislation that offer a variety of restrictions and requirements for pharmacy benefit managers (PBMs).
Sixteen states have banned a pharmacy benefit management practice that involves not counting the value of drug copay assistance from manufacturers toward patient deductibles.

Spending on specialty drugs fueled a 11% increase in Medicaid drug spending from 2020 to 2021, says Magellan's seventh annual Medicaid Pharmacy Trend Report.

Biosimilars will have limited effect on the oncology drug expenditures, according to IQVIA. Spending on obesity drugs may increase tenfold, depending on guidelines and payer coverage.

Spending using net prices (prices after discounts and rebates are factored in) will grow between -1% and 2% between 2023 through 2027, say IQVIA analysts in the company's Global Use of Medicines report. Patent expiration and competition from generics and biosimilars and the healthcare-related provisions of the Inflation Reduction Act will push down spending, predict IQ.

Manufacturers and payers will now be allowed to exchange information before a drug is approved by FDA.

Baker discusses specialty drugs and their discounting in this segment of his interview with Peter Wehrwein, managing editor of Managed Healthcare Executive.®

Baker spoke with Peter Wehrwein, managing editor of Managed Healthcare Executive®, about the smaller, upstart pharmacy benefit managers (PBMs) and their role in the industry. Some are using newer technology as their calling card, he says.
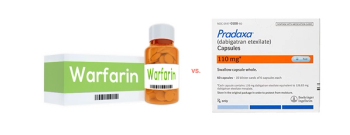
Pradaxa (dabigatran) and Coumadin (warfarin) came out even in this randomized study. The Brazilian researchers who conducted the study said one explanation for the result is that use of Coumadin was exceptionally well managed because the patients were enrolled a study.

Kareem Karara, Pharm.D., BCPS, CCHP, spoke about unbranded biologics, which are identical the branded products. They are coming on the market amid a growing number of biosimilars.

Agenda includes sessions on overall trends in the pharmacy benefit management industry, digital therapeutics and biosimilars.


The respondents were almost evenly split on whether it was a mistake for the FDA to approve Aduhelm, but a large majority agreed that CMS made the right decision to restrict Medicare coverage to those enrolled in clinical trials.

Opinion was split evenly on preexposure prophylaxis as a strategy for preventing HIV infections.

Two-thirds of the respondents to our 2022 annual Pharmacy Survey believe that biosimilars will have a significant effect on drugs prices over the next several years.