
COVID-19 care disruptions cut U.S. one-year cancer survival, adding 17,390 deaths; see which cancers and groups were hit hardest.

COVID-19 care disruptions cut U.S. one-year cancer survival, adding 17,390 deaths; see which cancers and groups were hit hardest.

FDA proposes MRD-negative and complete response endpoints to fast-track multiple myeloma drug approvals, pushing deeper molecular results over ORR.

PBM reform has finally made its way through Congress and was signed into law by President Donald Trump. Here are six things you need to know about the law

TrumpRx launched as a government-branded, direct-to-consumer discount platform for select brand-name drugs, but critics argue its cash-only savings may be limited, potentially misleading, and less cost-effective over time than traditional insurance or other programs.

Adolescent and young women are far less likely than men to receive HIV prevention medication despite meeting clinical eligibility and facing substantial HIV risk.

And what has it achieved? The meaning and achievements of managed care change with the times, the context, the goals — and in the eyes of the beholders.

Cigna reported 11% revenue growth for 2025. Company executives focused on several key issues in its call with investors: its settlement with the Federal Trade Commission, a transformation announced last year to move to a rebate-free model in pharmacy benefits, and a focus on patient affordability.

A meta-analysis of more than 20 phase 3 trials found no clear survival differences by sex or age in metastatic NSCLC but reported better outcomes for Asian patients and continued underrepresentation of Black patients.

Express Scripts agreed to overhaul its business model, ensuring patients pay the lowest available price for medications and reimbursing community pharmacies based on actual costs.

Massachusetts leads SmileHub’s women’s health ranking, revealing cost barriers, safety gaps and state policies that shape care and outcomes.

Study finds transfusion access drives hospice choices in advanced blood cancers, urging new models that offer palliative transfusions with comfort care.

PBM critics heaped praise on reforms. A communications official at the trade group should turn its attention to how the pharmaceutical industry stifles competition and keeps prescription drug prices high.

Recent research compares sleep aids in apps—sonic sleep sounds, white noise and pink noise—and warns weak evidence may waste money and delay proven insomnia care.

Rural hospital study found text messaging intervention significantly improved medication adherence for heart failure patients, demonstrating that simple technology can reduce readmissions.
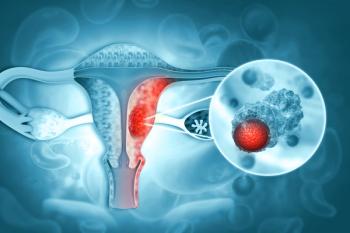
A large Korean, population-based study of more than 2 million women suggests that patterns across a woman’s reproductive life may influence ovarian cancer risk, with some factors increasing risk and others appearing protective.

Patients using GLP-1 medications may notice facial changes due to fat loss. Vasyukevich stresses education and options for maintaining a youthful appearance.
The award will involve patients with chronic disease and their families to inform clinical research, with an initial focus on diabetes.
Estimates show melanoma diagnoses continuing to climb in the United States, even as newer therapies continue to reduce deaths from advanced disease.

Myqorzo was approved in December to treat adults with obstructive hypertrophic cardiomyopathy. It is available through a REMS program and has a boxed warning about the risk of heart failure.



Ten Medicaid tech companies pledge over $600 million in support to help states meet new community engagement requirements and modernize eligibility systems.
A recent study found that older adults who received the shingles vaccine were biologically younger than those who didn’t, which suggests the vaccine may slow aspects of biological aging as well as prevent shingles.

Explore the impact of ACA tax credits on enrollment, premiums and healthcare access as Congress debates subsidy extensions for 2026 and beyond.

Yuvezzi is the first combination product that treats adults with presbyopia. It will be available in the second quarter of 2026.

Urgent care spending surges over 50% from 2018 to 2022, driven by increased visits, highlighting its vital role in healthcare access.

Although Medicaid expansion has been linked to lower mortality, significant racial disparities persist.

CMS announced 15 drugs for Medicare price negotiations under the IRA, representing $27 billion in spending and treating conditions such as diabetes, cancer, and autoimmune conditions. Here are three insights on the list from industry experts.