
The FDA has accepted the biologics licensing application (BLA) resubmission for odronextamab, a potential treatment for relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma.


The FDA has accepted the biologics licensing application (BLA) resubmission for odronextamab, a potential treatment for relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma.

Merilog is expected to be available in July 2025, and Sanofi will provide Merilog to patients for $35 or less for a 30-day supply.

Lenacapavir is a twice-yearly injectable medication designed to be used as pre-exposure prophylaxis (PrEP). The FDA is giving this drug a priority review and expects to make a decision by June 19, 2025, according to a release.
So far, five biosimilars that reference Eylea 2 mg have been approved by the FDA. Alvotech/Teva expect to regulatory approval for its biosimilar to be completed in the fourth quarter of 2025.

Samsung Bioepis’ biosimilars Ospomyv and Xbryk have been approved to be interchangeable with Prolia/Xgeva treat osteoporosis and multiple myeloma/bone metastases from solid tumors.
Gomekli is the second ever FDA-approved treatment for rare tumor disease, NF1-PN and the first to be approved for both adult and pediatric patients.
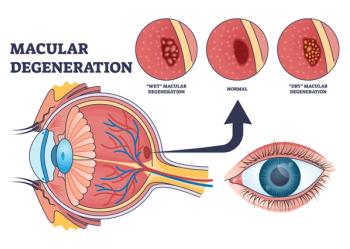
The updated label allows physicians to prescribe Izervay without a limitation on duration for patients with geographic atrophy secondary to age-related macular degeneration.

The PDUFA action date for an FDA decision for linvoseltamab to treat adult patients with relapsed/refractory multiple myeloma is July 10, 2025.

Emblaveo will be available in the third quarter of 2025 to treat patients with intra-abdominal infections, a common cause of sepsis in the intensive care unit.
Regulators have set a goal of Aug. 12, 2025, for brensocatib to treat patients with non-cystic fibrosis bronchiectasis, a chronic lung disease.
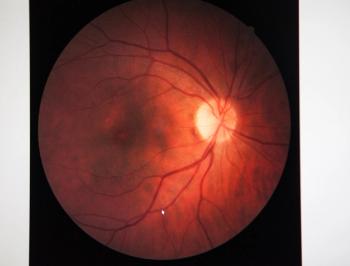
Susvimo is also approved to treat age-related macular degeneration, and the medication, ranibizumab, is delivered though a refillable ocular implant.

The new biosimilar, Celltrion’s Avtozma, was approved in both IV and subcutaneous forms to treat inflammatory conditions such as arthritis.
As a non-opioid drug, Journavx can assist patients effectively and without showing signs of being addictive.

Chronic kidney disease affects about 37 million adults in the United States and is expected to rise. It is a common complication of type 2 diabetes.

A monthly 10 mg/mL maintenance dose of Leqembi had the same effects on mild Alzheimer’s symptoms as biweekly dose, research shows.

This new generic medicine is designed to be a simple, once-daily treatment for children as young as three months old who weigh at least 13 pounds.
An investigational higher dose of spinal muscular atrophy drug nusinersen gains attention as the FDA and European Medicines Agency (EMA) considerate it as an alternative than the current lower FDA-approved dosage.

TriVerity focuses on whether the patient has an infection and how severely ill they are likely to become.
The investigational advanced melanoma combination therapy consisting of RP1 and nivolumab have a PDUFA action date of July 22, 2025.

One-third of folks with major depressive disorder experience treatment-resistant depression (TRD), a condition that makes it more difficult to be treated by traditional oral antidepressants.
Datroway reduced disease progression by 37% when compared with chemotherapy.
More than half (53%) of patients reached remission after one year of Omvoh treatment and 46% experienced healing of the intestinal tract, when compared to placebo.
If approved, subcutaneous autoinjector Leqembi will be the only Alzheimer's medication that can be administered at home. The FDA has set a goal date of Aug. 31, 2025.

The target action date for Rexulti in combination with sertraline to treat patients with post-traumatic stress disorder, originally planned for Feb. 8, 2025, will be delayed.
Kerenida is currently approved to slow the progression of chronic kidney disease associated with type 2 diabetes.