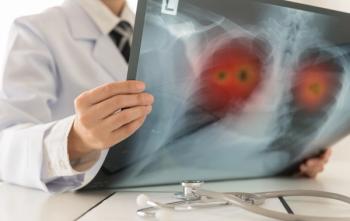
Ibtrozi had a 90% response rate in treatment naïve adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer, according to the results of a news release.

Ibtrozi had a 90% response rate in treatment naïve adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer, according to the results of a news release.

Enflonsia is the first FDA-approved respiratory syncytial virus (RSV) preventative for infants, regardless of weight, according to the news release.
Sibeprenlimab would be offered in a prefilled syringe for subcutaneous injection. The PDUFA target action date is Nov. 28, 2025.
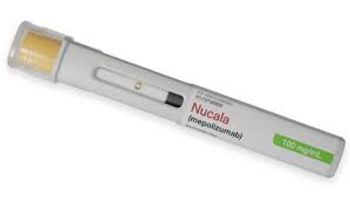
Nucula’s new indication is as an add-on therapy for patients with COPD who also have an elevated blood eosinophil count.
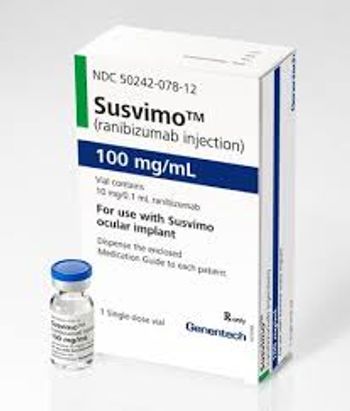
Susvimo is also approved to treat diabetic macular edema and age-related macular degeneration, and the medication, ranibizumab, is delivered through an ocular implant that is refilled every nine months.
Bayer’s Jivi is a factor VIII replacement therapy that has been engineered with a compound that allows for less frequent dosing. It is now indicated for patients 7 years of age and older.

Zynyz is now FDA-approved as the first and only approved first line treatment for advanced squamous cell carcinoma of the anal canal.
If approved, Wegovy would be the first oral GLP-1 drug to treat obesity.

Adult and pediatric generalized myasthenia gravis patients have a new, longer acting option for treatment called Imaavy.

The complete response letter did not identify any issues with the safety or efficacy of Eylea HD, Regeneron officials said.
Chronic spontaneous urticaria is chronic inflammatory skin disease driven in part by type 2 inflammation, which causes sudden and debilitating hives and recurring itch.
If approved, Eylea would be the first treatment for RVO with 8-week dosing. The FDA target action date is Aug. 19, 2025.

Jobevne is the fifth Avastin biosimilar, which are recombinant humanized monoclonal antibodies used to treat several different types of cancer.
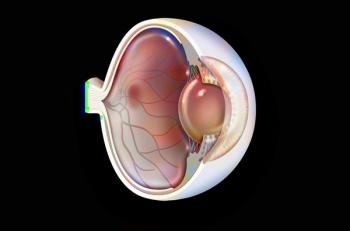
The FDA has set a goal date of Aug. 27, 2025. If approved, the therapy would be branded as Lytenava and be the first ophthalmic formulation of bevacizumab.
Uplizna is the first approved treatment for immunoglobulin G4-related disease (IgG4-RD), a chronic inflammatory condition that can affect multiple organs. It has a price of $140,248.50 per dose.
Vanrafia reduces proteinuria in adults with primary immunoglobulin A nephropathy (IgAN). It has a wholesale acquisition cost of $162,500 annually.

The fourth pair of denosumab biosimilars, Conexxence and Bomyntra, are expected to launch in the United States in mid 2025, as a result of a global settlement with Amgen, according to a company news release.

Vykat XR will be available in April to treat the intense hunger that is a hallmark of the rare genetic disease Prader-Willi syndrome. The price is based on a patient’s weight, and the average patient in the clinical trials would have had an average annual cost of $466,200 for the first year.
Neuroendocrine tumors that start in the pancreas tend to be more aggressive and have a poor prognosis.

This expanded indication for Amvuttra makes it the first and only FDA-approved treatment for transthyretin amyloidosis with cardiomyopathy (ATTR-CM) and the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR-PN) in adults.

Tremfya is also approved to treat patients with plaque psoriasis, active psoriatic arthritis and ulcerative colitis and is available as both a subcutaneous and intravenous induction option.
This is the third approval for Fabhalta (iptacopan), which was discovered and developed by Novartis.

Regulators are expected to make a decision in the third quarter of 2025 on Kerendia to treat patients with heart failure with a left ventricular ejection fraction (LVEF) of ≥40%.
Two companies have received approved for a generic of the 2.5 mg tablet of anticoagulant rivaroxaban, which is used to reduce the risk of stroke and deep vein thrombosis.

Celltrion’s Omlyclo is interchangeable with Xolair to treat the same conditions: asthma, chronic rhinosinusitis, food allergy and chronic spontaneous urticaria.