
Duvyzat was approved in March 2024 and works to reduce the inflammation and loss of muscle experienced by patients with Duchenne muscular dystrophy.

Duvyzat was approved in March 2024 and works to reduce the inflammation and loss of muscle experienced by patients with Duchenne muscular dystrophy.
Epysqli is a monoclonal antibody that is approved to treat two rare conditions that break down red blood cells.
If approved, tabelecleucel would be the first therapy specifically to treat Epstein-Barr virus related post-transplant lymphoproliferative disease. The FDA’s action date is Jan. 15, 2025.

OX124 is a nasal spray provides rapid absorption of naloxone for patients experiencing an opioid overdose. The FDA would like to see additional technical data, as well as data on whether patients can correctly use the device.
Giant cell arteritis is an inflammatory disease that affects the large blood vessels that supply blood to the head and brain.
The FDA is asking for information about the manufacturing process, as well as the type 1 diabetes indication.
Genentech had recalled the Susvimo ocular implant two years ago. The FDA has approved changes to the implant and needle.
The prefilled syringe of Vabysmo will become available in the coming months. It treats age-related macular degeneration, diabetic macular edema and macular edema following retinal vein occlusion.

Donanemab — now with the brand name of Kisunla — slows cognitive and functional decline by up to 35% and has a list price of $695.65 per vial.

Samsung Bioepis’ Pyzchiva (ustekinumab-ttwe) will be available beginning Feb. 22, 2025 and will be marketed by Sandoz.
For the second time, the FDA is asking for additional information about chemistry manufacturing and controls.
Ohtuvayre - an inhaled bronchodilator with non-steroidal anti-inflammatory effects - is the first new mechanism in COPD approved in more than 20 years.
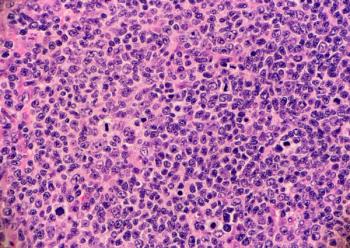
Epkinly is a bispecific antibody now approved to treat both relapsed or refractory follicular lymphoma and relapsed or refractory diffuse large B-cell lymphoma.

The FDA cited issues related to a third-party manufacturer. The agency did not request any additional testing from AbbVie.
Krazati is already available to treat patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer.

Full approval was granted for the one-time gene therapy to ambulatory patients aged 4 years older. The FDA also granted accelerated approval to Elevidys for non-ambulatory patients.

Skyrizi is also available to treat patients with plaque psoriasis, psoriatic arthritis, and Crohn’s disease and has a list price of $21,017.36 for one dose.

Committee members, however, also said more data are needed on donanemab to treat patients in underrepresented patient groups, including Latin American and African American patients and special populations such as Down syndrome.
Tagrisso, which is already available to treat patients with non-small cell lung cancer and EGFR mutations, is being reviewed by the FDA for patients with this cancer and exon 19 deletions or exon 21 mutations.
The FDA is considering a monthly IV maintenance dose of Leqembi to treat people with Alzheimer’s disease. The action date is set for Jan. 25, 2025.

mRESVIA will be available for the 2024/2025 RSV season as a pre-filled, ready to use syringe.
Zolbetuximab is first-in-class monoclonal antibody. The FDA has assigned an action date of Nov. 9, 2024.

FDA officials have asked for additional analyses on the efficacy of Dupixent in the two pivotal trials. The revised target action date is Sept. 27, 2024.
Breyanzi is a CAR T-cell therapy now approved for four subtypes of non-Hodgkin lymphoma.

Onyda XR is a liquid form of clonidine that can be used as monotherapy or as adjunctive therapy for patients 6 years of age and older with ADHD.