
If approved, Awiqli would become the first once-weekly basal insulin available in the United States for adults with Type 2 diabetes.

If approved, Awiqli would become the first once-weekly basal insulin available in the United States for adults with Type 2 diabetes.
Evkeeza is now approved for the treatment of homozygous familial hypercholesterolemia (HoFH) in patients as young as 1 year old.
Tavapadon is an investigational, novel, once-daily treatment option for Parkinson’s disease, which currently affects more than 1 million Americans.

FDA rejects Rexulti's approval for PTSD treatment, citing insufficient evidence of effectiveness in combination with sertraline. Otsuka and Lundbeck announced they are planning next steps.
The FDA letter requested that a technical information update be included in the Chemistry Manufacturing and Controls (CMC) module of the sNDA.

The complete response letter was issued by the FDA during a routine inspection of a third-party fill-finish facility, not because of an issue with apitegromab.

Tremfya is now the first and only fully subcutaneous IL-23 inhibitor approved to treat both ulcerative colitis and Crohn’s disease.

This spray formulation of bumetanide demonstrated a 33% faster absorption rate than oral bumetanide, according to clinical trial data.
Leqembi IQLIK, a subcutaneous version of Leqembi, will launch on Oct. 6, 2025, with a list price of $375 per autoinjector.
Liraglutide, a generic of Saxenda, is approved to treat adults and adolescents who are obese or overweight.

Orforglipron will be submitted to global regulatory authorities for the treatment of obesity or for weight loss by the end of 2025, with diabetes submissions to follow in 2026.
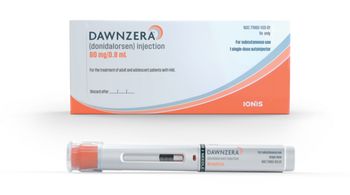
Dawnzera (donidalorsen) will be available in a few days with a list price of $57,462 per dose

The FDA has granted priority review of the sNDA and assigned a goal date of Dec. 20, 2025, for Imcivree to treat patients with acquired hypothalamic obesity, which is caused by an abnormality of the hypothalamus.
Tonmya is the first new fibromyalgia treatment approved by the FDA in more than 15 years, providing symptom relief for at least 30% of patients when compared to placebo, according to clinical trial results.
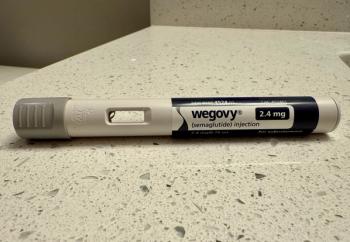
Wegovy is the first GLP-1 drug approved to treat adults with metabolic dysfunction-associated steatohepatitis (MASH). An ongoing study aims to confirm the benefits.

Papzimeos is the first and only FDA-approved treatment for adults with recurrent respiratory papillomatosis (RRP), with ongoing clinical studies showing symptom relief for up to two years.
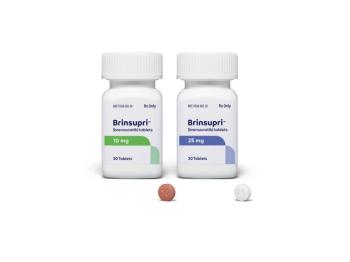
Brinsupri is the first approved therapy to address the underlying inflammatory process of non-cystic fibrosis bronchiectasis (NCFB).

Teva's Ajovy gains FDA approval for pediatric migraine prevention, offering hope for children aged 6-17 with this debilitating condition.
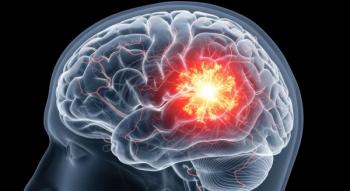
Modeyso is the first drug to treat patients with diffuse midline glioma with an H3 K27M mutation, an ultra-rare and aggressive brain tumor.

The FDA has approved VIZZ, a once-daily, preservative-free eye drop that improves near vision in adults with presbyopia for up to 10 hours.

Sarepta continues to work with regulators to complete the safety label update for Elevidys, and they are discussing an approach for risk mitigation for non-ambulatory patients.
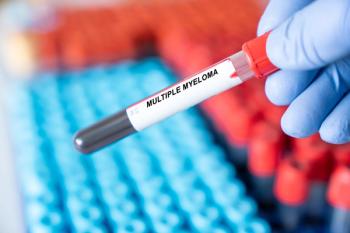
Last week, an FDA advisory committee against the risk-benefit profile of Blenrep in combination with other therapies. Regulators and reviewers were concerned about the ocular side effects and dosing and tolerability. The new action date is Oct. 23, 2025.

Sarepta officials said the temporary halt in shipments was done to maintain a productive working relationship with regulators while they address a safety labeling update about the risk of acute liver disease related to Elevidys.

Elevidys is a gene therapy approved to treat Duchenne muscular dystrophy. Last month, Sarapta halted sales of the therapy for non-ambulatory patients.

Orsini will be the specialty pharmacy partner for Ekterly.