
The UC Davis team is studying a less invasive way of delivering gene therapy to the eye: injecting the gene therapy into the suprachoroidal space, a tiny layer between the white of the eye and the blood vessels that feed the retina.

The UC Davis team is studying a less invasive way of delivering gene therapy to the eye: injecting the gene therapy into the suprachoroidal space, a tiny layer between the white of the eye and the blood vessels that feed the retina.

To help Medicare plans better manage members with high drug expenses, Prime Therapeutics has launched an AI-driven tool to help plans identify members likely to experience increases in pharmacy costs.

Managing specialty and cancer drugs, as well as the GLP-1 therapies, tops the list of priorities this year and next, according to a survey by the International Foundation of Employee Benefit Plans.

In this second part of a video interview series, it was revealed that Sharon Faust, chief pharmacy officer at Navitus Health Solutions and PBMI Innovator Award winner, works to improve the pharmacy benefit system by promoting transparency, educating others about rules and supporting new business ideas that help patients and use healthcare money wisely.
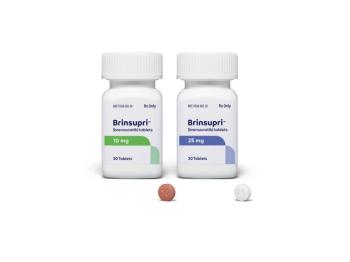
Brinsupri is the first approved therapy to address the underlying inflammatory process of non-cystic fibrosis bronchiectasis (NCFB).

Specialty drug spending rose 9.6% in 2024, but Humira biosimilar adoption is helping to slow cost increases.

The American Society of Retina Specialists’ History of Retina is an initiative that highlights the milestones and the pioneers in ophthalmology and retinal disease treatment.

Joshua Fredell, Pharm.D., senior vice president at CVS Caremark and PBMI Innovator Award winner, is calling for a PBM industry focus on making pharmacy experiences more seamless while continuing to keep drug costs affordable.

Senior vice president at CVS Caremark
Researchers say that a deficiency in the brain of the hormone leptin is as much a part of the cascade that can lead to diabetic ketoacidosis as a lack of insulin is. This opens the potential for the development of new therapies for patients with Type 1 diabetes that target the brain.
Modeyso is the first drug to treat patients with diffuse midline glioma with an H3 K27M mutation, an ultra-rare and aggressive brain tumor.
Ixchiq now has a warning about its use among elderly people with multiple underlying health conditions after postmarketing reports indicated there is a risk of neurologic and cardiac events in people over the age of 60.

In a video interview with MHE editors, Nathan Downhour, Pharm.D., vice president of strategic pharmacy solutions at Prime Therapeutics, said a shift is already underway in the pharmacy benefit market in terms of becoming more transparent and sustainable across the industry.

Senior vice president of strategic pharmacy solutions, Prime Therapeutics

Founder, chairman and CEO, LucyRx
Farxiga, along with insulin and monitoring for diabetic ketoacidosis, shows promise for kidney and glucose control in youth with Type 1 diabetes.

Two presentations discussed the significant care disruptions when there are copay assistance shortages, with patients forgoing effective treatments and turning to lower-cost alternatives with worsening outcomes.

A new analysis finds that prior authorization for anti-VEGF therapies increases societal costs despite minimal insurer savings, burdening patients, workplaces, and providers.
Ixo-vec reduced anti-VEGF injection frequency and was well tolerated in patients with wet AMD. A phase 3 pivotal trial comparing it with Eylea 2 mg is under way.

IRX-101, a new antiseptic tested in patients receiving eye injections for macular degeneration, resulted in less pain and fewer adverse events than povidone-iodine.

In a study in mice, a wireless implant was able to release glucagon as an emergency treatment for hypoglycemia, a serious complication of Type 1 diabetes.
Anti-VEGF and anti-complement therapies work on distinct mechanisms in age-related macular degeneration, and both may be required to treat patients with advanced disease or complex cases.
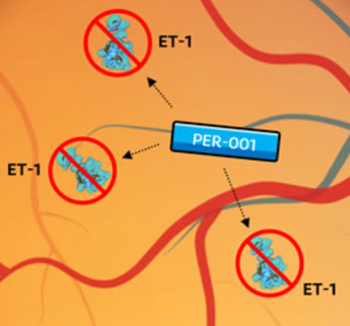
PER-001, delivered through a slow-release, dissolvable implant in the vitreous cavity of the eye, is designed to block endothelin signaling to increase ocular blood flow and prevent vision loss.
C3 glomerulopathy (C3G) and immune-complex membranoproliferative glomerulonephritis (IC-MPGN) are two diseases that have a high risk of kidney failure. Empaveli is also approved to treat patients with paroxysmal nocturnal hemoglobinuria.

Sarepta continues to work with regulators to complete the safety label update for Elevidys, and they are discussing an approach for risk mitigation for non-ambulatory patients.

Two PBMs, True Rx Health Strategies and Capital Rx, are using pharmacogenomics — how a person’s DNA affects their response to medications — to reduce the trial-and-error of prescribing medications, saving employers and patients time and money.
Health policy researcher Geoffrey Joyce argues that only delinking compensation from the list price of a drug will lower drug spending.
Last week, an FDA advisory committee against the risk-benefit profile of Blenrep in combination with other therapies. Regulators and reviewers were concerned about the ocular side effects and dosing and tolerability. The new action date is Oct. 23, 2025.

The Vision Center at Children’s Hospital Los Angeles is developing a protocol for a phase 1 clinical trial for the first gene therapy for boys with blue cone monochromacy, which impairs color vision and severely affects visual function.

Sarepta officials said the temporary halt in shipments was done to maintain a productive working relationship with regulators while they address a safety labeling update about the risk of acute liver disease related to Elevidys.