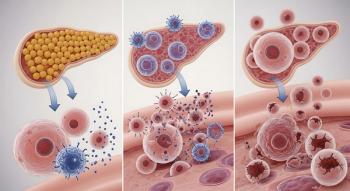
Islet Cell Therapy Has Potential to Cure Type 1 Diabetes
After a one-year follow-up, 10 of 12 patients given the islet cell therapy zimislecel no longer required insulin. Vertex officials said regulatory submissions are expected in 2026
All 12 patients with Type 1 diabetes who have received Vertex Pharmaceuticals’ zimislecel and with at least one year of follow-up have achieved A1C levels less than 7% with no severe hypoglycemic events, a potentially life-threatening low-blood-sugar condition. These data were recently presented at the 85th Scientific Sessions of the American Diabetes Association meeting and also
Type 1 diabetes is an autoimmune disease where the immune system destroys insulin-producing beta cells in pancreatic islets. Insulin deficiency results in hyperglycemia and can lead to acute life-threatening complications such as diabetic ketoacidosis, a serious complication where the body is not able to use sugar for energy. Patients rely on insulin and careful monitoring of blood glucose levels, but if blood sugar drop . About 2 million Americans have Type 1 diabetes.
Zimislecel is an investigational allogeneic, or donor, stem cell-derived islet cell therapy that is delivered by an infusion into the liver portal vein. The therapy aims to replace the insulin-producing cells that have been destroyed in people with Type 1 diabetes.
Vertex officials estimate that about 125,000 patients have severe Type 1 diabetes, and if approved for this indication, zimislecel could be appropriate for approximately 60,000 people with severe Type 1 diabetes.
“As I think about my patients and the unmet need in the Type 1 diabetes community, the results we’ve seen so far for restoring endogenous insulin secretion with a stem cell-derived islet therapy bring me hope and confidence for a transformative treatment option for individuals with Type 1 diabetes in the not-so-distant future,” Michael R. Rickels, M.D., M.S., medical director, Pancreatic Islet Cell Transplant Program, Willard and Rhoda Ware Professor in Diabetes and Metabolic Diseases in the Perelman School of Medicine at the University of Pennsylvania, said in a
The single-arm, open-label
For this analysis, investigators found that all 12 participants achieved a reduction in A1C of less than 7% and were able to reduce their use of insulin. Of the 12 patients, 10 patients no longer required insulin. Additionally, all 12 patients had no severe hypoglycemic events after the first 90 days.
The majority of adverse events were mild or moderate and included diarrhea, headache, nausea, COVID-19, mouth ulceration, neutropenia and rash. Most of the treatment-related adverse events were attributed to the immunosuppressive therapy given to patients to prevent rejection of the islet cells.
Vertex expects to complete enrollment and dosing of about 50 patients this year, with regulatory submissions to the FDA, the European Medicines Agency and the UK Medicines and Healthcare Products Agency in 2026.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.