Bispecific T-cell engagers demonstrate lower total costs and reduced cytokine release and neurotoxicity rates compared with CAR T therapy for patients with follicular lymphoma.

Bispecific T-cell engagers demonstrate lower total costs and reduced cytokine release and neurotoxicity rates compared with CAR T therapy for patients with follicular lymphoma.

Payers and providers are using real-world data to identify high-performing treatment centers, reduce administrative bottlenecks, and support value-based contracting arrangements.

Former House Speaker Paul Ryan called for evidence-based reforms, risk pools and premium support models for Medicare, to fix the nation’s strained healthcare system.

Panelists at AMCP’s policy summit debated PBM reform, disagreeing on spread pricing ethics and rebate transparency while discussing costs, risks, and whether current contracting models benefit patients.

"It’s people versus business interests, party interests, and ideology," Robert Costa of CBS News told the AMCP Nexus audience.

Benjamin Lockshin, M.D., from Georgetown University, discusses how oral TYK2 inhibitors that rival biologics’ efficacy have changed the management of patients with psoriasis.
New clinical data demonstrate Zoryve's effectiveness in treating atopic dermatitis, reducing sleep disruptions and maintaining disease control with minimal adverse events across all ages.
Icotrokinra is a first-in-class targeted oral peptide that blocks interleukin (IL) 23 and its receptor, which plays a key role in the inflammatory response in plaque psoriasis.

James Song, M.D., discusses IL-23 inhibitors’ drug persistence in psoriasis treatment, new systemic therapy criteria, and upcoming oral medications that may rival biologics’ efficacy.

Foluso Agboola discusses the Institute for Clinical and Economic Review's newest analysis, which has found that most new drugs approved over a three-year period were priced above value benchmarks, potentially costing the healthcare system $1.5 billion.
Tzield, a monoclonal antibody approved for stage 2 Type 1 diabetes, now seeks approval for stage 3 disease based on trial data showing that it slowed beta cell loss in children and adolescents.

Conflicting studies on GLP-1 drugs’ vision impacts prompt calls for baseline eye exams and monitoring.

A study using AAO's IRIS Registry demonstrates Eylea HD 8 mg improves vision in treatment-naive patients with wet age-related macular degeneration or diabetic macular edema with previously treated patients extending dosing intervals two weeks.
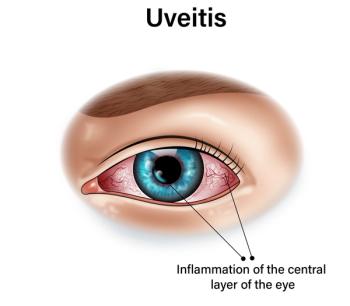
Patients receiving adalimumab for noninfectious uveitis also experienced fewer cataracts requiring surgery and better visual acuity outcomes compared with those on conventional immunosuppressive medications.

A new analysis has confirmed what doctors had said in a recent survey: loss of copay assistance for retina treatments can worsen vision as they switch to alternatives or delay treatment.

The new model offers direct manufacturer pricing for weight loss drugs and a way to contribute to employee costs.

A new California law prohibits spread pricing and requires all rebates to be passed to health plans and is an important step toward transparency across the entire pharmacy supply chain, said Blue Shield of California’s Kathy Chang in an interview.
A real-world analysis shows Shingrix effectively prevents shingles in adults over the age of 65, including immunocompromised people. Two doses provide better protection than one.
Children and adolescents who are infected with COVID-19 more than one time are at an increased risk of heart inflammation, blood clots, and other serious complications.
A recent study finds that despite biosimilar use rising to 60% to 86% for three cancer drugs, predicted savings don’t match those that would be achieved from a site-neutral reimbursement strategy.

Jascayd targets the enzyme PDE4B, which plays a role in the inflammatory process. The new therapy is able to affect both fibrosis and the immune system. It will be available in the next few weeks.

Pfizer only offered to discount six products through the Most Favored Nation program, and healthcare policy experts suggest the real-world impact remains uncertain.
In a recent trial, verapamil showed a trend toward preserving insulin-producing beta cells in patients with Type 1 diabetes but failed to achieve statistical significance.

As part of the Most Favored Nation pricing effort, Pfizer has agreed to invest in U.S. manufacturing and to allow access to its drugs at a discount through a new direct-to-patient website.

Novartis said that it is exploring an additional direct-to-employer model to increase access and affordability for cash-paying patients in the United States.

If approved, Awiqli would become the first once-weekly basal insulin available in the United States for adults with Type 2 diabetes.

AstraZeneca will launch a new online platform to make Airsupra, as well as Farxiga and Flumist, available for home delivery at a cash price that is up to 70% off the list price.
Investigators have identified the lowest dose of the immunosuppressive ATG needed to preserve beta cells in newly diagnosed children with Type 1 diabetes with the lowest side effects.
Increasing the cholesterol-lowering protein ApoM in mice improved retinal health and may be the key to slowing age-related macular degeneration in its earliest stages.

Brian K. Lee, Ph.D., of Drexel University Dornsife School of Public Health, says that despite the fact that some studies suggest there is link between acetaminophen use during pregnancy and neurodevelopmental disorders, there is a lack of evidence to support that conclusion.