
Some effects of poor sleep (fatigue, daytime dysfunction) resemble some symptoms of long COVID. As it turns out, infected women who were healthy sleepers before and early in the pandemic were less likely to report long COVID symptoms.

Some effects of poor sleep (fatigue, daytime dysfunction) resemble some symptoms of long COVID. As it turns out, infected women who were healthy sleepers before and early in the pandemic were less likely to report long COVID symptoms.

Briana Contreras, an editor with Managed Healthcare Executive, and Peter Wehrwein, managing editor of MHE, got to chatting with the newest editorial advisory board member, Jeffrey Casberg, who is vice president of Clinical Pharmacy at IPD Analytics.

Insurers are using prior authorization and other managed care strategies to control costs associated with the growing number of prescriptions, on- and off-label, for the weight-loss medications.
Embracing Eastern and Western medicine practices is crucial in delivering the best healthcare outcomes.

The new service reflects Uber Health’s initiative to address some of the industry’s greatest insecurities and deliver better experiences across the healthcare ecosystem.

Experts say the successes and failures of alternative payment models (APMs) over the past decade have taught us a few things.

FDA has announced evidence-based changes that will allow more individuals to donate blood without increasing the risk of HIV infection.

The deal, initially tentative, was announced Friday between the administration and those challenging the Affordable Care Act mandate.

In a clinical trial, researchers concluded that direct oral anticoagulants were non-inferior to low-molecular-weight heparin.

Alternative payment models are still coming into focus, but so far, they haven’t lived up to the high expectations.

In efforts to find the effectiveness of metformin, ivermectin and fluvoxamine, researchers of the study observed the medications in adults aged 30 to 85 years who were overweight or obese and had been diagnosed with COVID-19 within the last three days.

The FDA has made three approvals this week: a novel drug for dry eye disease, a new indication for Prevymis to prevent CMV disease in adult kidney transplant recipients, and another prostate cancer indication for Lynparza. The agency has also set target action dates for the first CRISPR gene edited therapy and a supplement indication for Jemperli for earlier treatment of endometrial cancer. Additionally, Janssen submitted sBLA for Carvykti for early treatment of myeloma.

Briana Contreras of MHE spoke with Leah Dewey of Cotiviti in this month’s podcast episode about what she’s seeing as the process of Medicaid Redetermination is well underway.

The company paved the way for prescription digital therapeutics.

Weekly surveillance monitoring, also known as rapid cycle analysis, of more than 245,000 COVID-19 mRNA vaccine doses did not detect a safety signal for any outcome 21 days after vaccination.

The retail giant’s initiative to offer attractive pay in every market they operate reflects in the pay raise of all associates. The average salary for pharmacists is now more than $140,000, not including bonuses and incentives. After investments within the optician and vision department, it’s expected the average wage for opticians to be more than $22.50 an hour.
Coherus will launch Yusimry in July with a list price of $995 per carton (2 x 40 mg/0.8 mL autoinjectors); “the lowest price announced to date of any adalimumab offering in the United States,” the company said.

Eligible members can enroll into these programs through Virta and Omada Health tools under Capital. The programs are personalized for Capital members on an easy-to-use app offering one-on-one health coaching, medical tools, community support, activity tracking and more.

The drugs must have full “traditional” FDA approval and patients need to be enrolled in a registry that collects post-approval data efficacy and safety when the drugs are used in clinical practice.

Understanding whether the prevention of RSV infection during infancy can reduce the risk of childhood asthma is key to designing successful and preventive strategies, preventing long-term childhood respiratory morbidity, and outlining healthcare policy measures.
Respiratory syncytial virus (RSV) can be deadly for the very young and the old and frail. GSK's vaccine will be marketed under the brand name Arexvy and Pfizer's under the name Abrysvo.
Miebo is the first and only prescription eye drop approved for dry eye disease (DED) that directly targets tear evaporation.

The head of a gastroenterologist group criticizes the company for having a "slapdash approach."
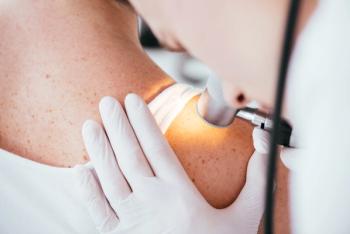
Lifileucel is a polyclonal tumor infiltrating lymphocyte (TIL) therapy designed for patients with advanced melanoma who have experienced progression after previous treatment with anti-PD-1/L1 therapy and targeted therapy.
Bowing to political and public pressure, insulin makers have slashed their prices. So far, insulin biosimilars haven’t had much of an effect on prices.

Editors are searching for diverse healthcare professionals from different backgrounds and healthcare sectors, with individual interests, and so on. Candidates should have, but not limited to, 12 years or fewer experience in healthcare.
Inpefa (sotagliflozin) is in the SGLT inhibitor class recommended as first-line treatment for heart failure by the American Heart Association and other groups. Jardiance (empaglifozin), a competitor in that class, has a list price of $570.48 for a month's supply.

Dated notions and lack of an early connection to healthcare keep men from seeking care.

The Biden-McCarthy leaves $5 billion intact for coronavirus vaccine and treatment research, according to multiple media reports.

The World Health Organization shared its enthusiasm for the “appropriate” use of these technologies. However, they are calling for caution to be exercised to protect and promote human well-being, safety, and autonomy and preserve public health.