The 200-patient trial will test whether adding a monoclonal antibody called itolizumab to high doses of corticosteroids will improve outcomes for patients who develop acute graft-versus-host-disease after a stem cell transplant.

The 200-patient trial will test whether adding a monoclonal antibody called itolizumab to high doses of corticosteroids will improve outcomes for patients who develop acute graft-versus-host-disease after a stem cell transplant.

Analysis of answers to online survey shows that telehealth use is high among people with multiple sclerosis (MS) amid the COVID-19 pandemic. Telehealth may be an appealing option for patients with MS who have physical impairments that make traveling to in-person appointments difficult.

The decades-long quest for a vaccine against HIV has been fruitless so far. Moderna hopes an HIV vaccine that uses its messenger RNA technology will break the losing streak. A phase 1 trial designed to include 56 volunteers has started.
Once approved, the insulins will be available to patients for no more than $30 per vial.

Physicians who are reluctant or even opposed to broad use of pre-exposure prophylaxis (PrEP) against HIV may keep physician assistants and nurse practitioners from prescribing the antivirals that can prevent infection and spread of HIV. Making PrEP an over-the-counter treatment would be one way of increasing access.
A recent meta-analysis shows that a large proportion of people with multiple sclerosis have osteopenia or osteoarthritis. Lack of mobility and vitamin D deficiencies may play a causative role.

Coverage requirements may result in premium hikes next year, say some health plan executives.
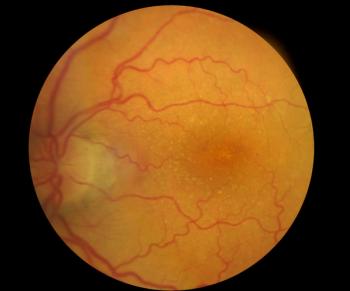
Off-label Avastin, Eylea and Lucentis are the primary treatments for neovascular age-related macular degeneration (AMD), a leading cause of blindness. A recent review article looked at the impediments to the biosimilars for these drugs Ophthalmologists are wary about using biosimilars to Avastin (which was approved as a cancer drug) for AMD. Biosimilars to Eylea and Lucentis are not on the market yet. Manufacturers’ rebates and Medicare Part B “buy and bill” policies could make it difficult for them to compete against their brand-name “originator” products.
When managed care got started in the early ’80s, the focus was on physician and hospital services. Primary care doctors were positioned as gatekeepers, controlling referrals to specialists. Insurers came under a lot of criticism for shortening hospital stays. Now much of the managed care energy is trained on drug costs.
How successful accountable care organizations (ACOs) have been in bringing about value-based care hinges, of course, on how success is defined. CMS’ comprehensive end-stage renal disease (ESRD) care model is a good example.

Like the entirety of the world, the COVID-19 pandemic exposed many issues in the medical industry. One area in particular: the clinical trials space developed many challenges as a result to the height and continuous battle of the pandemic.
The interplay between nonalcoholic fatty liver disease and diabetes can amplify both conditions, say two prominent researchers.

The setback for GlaxoSmithKline comes amid a multicompany race to develop a vaccine against respiratory syncytial virus infections.

Based on several reports in the last couple of years, it’s been a standout that healthcare consumers’ engagement in their own healthcare will drive better outcomes and reduce care costs. With that being said, consumer experience has come under major focus across the industry as a key business driver.

One of the priorities of the new institute at Virginia Commonwealth University’s medical school will be research into nonalcoholic steatohepatitis (NASH) as a key component in the emerging construct of metabolic health, says its leader, Arun J. Sanyal, M.D.
FDA approved Legend Biotech’s chimeric antigen receptor T-cell (CAR-T) treatment, Carvykti (ciltacabtagene autoleucel; cilta- cel), to treat relapsed or refractory multiple myeloma (RRMM) who have received four or more prior lines of therapy.

Briana Contreras, editor of Managed Healthcare Executive spoke with Jason Warrelmann, global director of Healthcare and Life Sciences at UiPath, in this week's episode of Tuning In to the C-Suite podcast. In the discussion Jason addressed the incline of automation in healthcare, why it's necessary for patients and providers and how other healthcare organizations can better overcome the challenges of adopting automation within their health system.
Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
Guidelines tell prescribers and patients to avoid switching among levothyroxine products from different manufacturers. But Mayo Clinc-led research finds little difference between switchers and nonswitchers.
In the final part of a two-part video series, Wes Gilson, senior director MR of Business Development at Siemens Healthineers, advises how payers and health facilities can help improve patient access to MRI care, and shares some MRI alternatives.

CDC figures from June 2020 show that about 41% of adults had opted to delay visiting a medical provider. Now, 20-plus months later, care avoidance of common chronic and acute conditions has only worsened.

Ideally, value-based and technology programs combine to produce better outcomes. But researchers at University of California, San Francisco, found little evidence of synergy in the meaningful use, patient-centered medical home and Medicare Shared Savings Program ACO program.

The new indication for reducing the risk of cardiovascular death and hospitalization for heart failure is expected to boost Jardiance’s sales further. Revenue for the drug jumped 38% in 2021 to reach nearly $432 million globally.
Colorectal cancer disparities between Black and white adults were eliminated among Kaiser Permanente members in Northern California after the healthcare organization instituted a regionwide, structured colorectal cancer screening program.

In this first part of a two-part video series, Wes Gilson, senior director MR of Business Development at Siemens Healthineers, addresses why improving access to magnetic resonance imaging (MRI) is crucial to advancing health equity, and what barriers are limiting that access to patients.
This guidance discusses that individuals 12 years and older can receive the second dose of the Pfizer-BioNTech vaccine 3-8 weeks after the first. Additionally, the interval for those 18 years and older for Moderna is 4-8 weeks.

A recent study found 70% of patients believe remote patient monitoring enables better care management.

In discussing company’s drug spend report, CVS Caremark President Alan Lotvin, M.D., bats away criticism of the pharmacy benefits management (PBM) industry, blames drugmakers for high drug prices, and says independent pharmacies are doing well because of participation in “humongous buying groups.”

Most Health plans and much of healthcare, overall, have had their feet on the gas when it comes to more patient-centered care or consumer choice services. COVID-19 has clearly made it all about the consumer in many ways, but more specifically, in making care much more accessible.