Eyecare
Latest News
Latest Videos
CME Content
More News

Experts say ordinary sunglasses aren't protective enough for viewers of the solar eclipse on Monday. You need those certified eclipse glasses. Ten-packs were selling on Amazon today for $12.99.
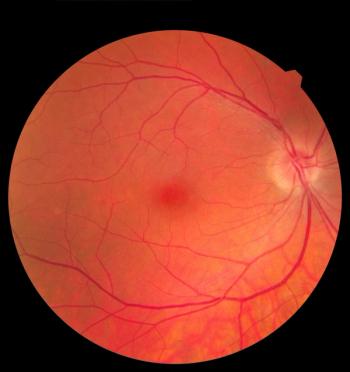
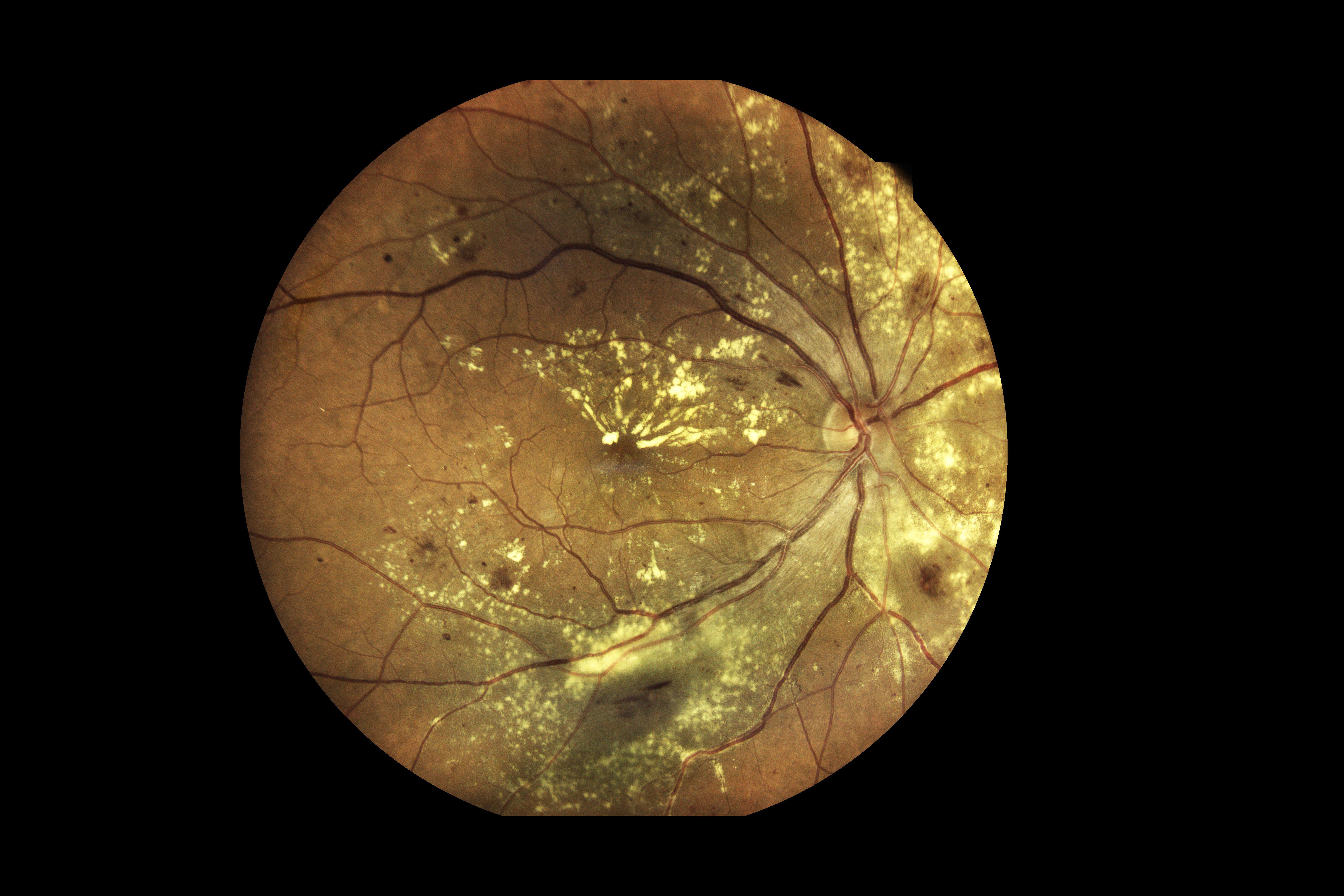
The AI system correctly identified all of the severe cases and accurately detected 80% of the cases with more-than-mild retinopathy of prematurity in a real-world population.
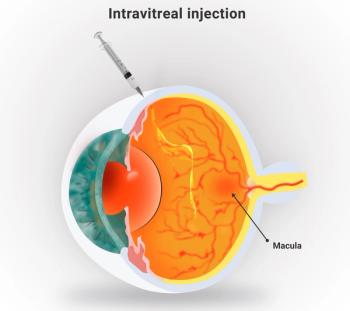
Vision symptoms resolved within a month, the specialists said in their report in JAMA Ophthalmology, but they say that retina specialists need to be aware that ths side effect from Vabysmo (faricimab) is more common that data from clinical trials would indicate.
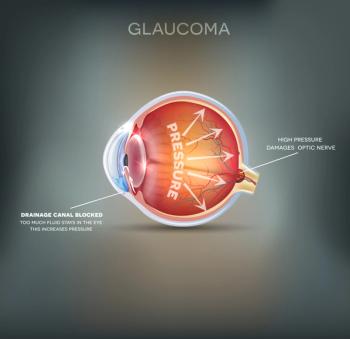
iDose TR was approved to reduce intraocular pressure in patients with ocular hypertension or open-angle glaucoma. It has a wholesale acquisition cost of $13,950 per dose/implant.

Researchers have identified an association between cataract surgery and a lower risk of cognitive decline and dementia.

Johns Hopkins researchers investigated how home environments may affect physical activity.

Glaucoma is a leading cause of blindness, and African Americans and other minorities have a higher risk for the disease.
Outlook Therapeutics plans to begin a study in the first quarter of 2024 to address the issues identified in a FDA complete response letter. If approved, Lytenava would be the only bevacizumab product to specifically treat age-related macular degeneration.
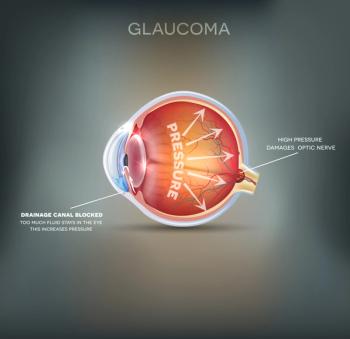
IDose TR will be available in first quarter of 2024 with a wholesale acquisition cost of $13,950 per dose/implant.

A California retinal specialist argues in a JAMA Ophthalmology viewpoint article that an independent panel of retinal specialists and statisticians should assess the data from clinical trials of Syfovre (pegcetacoplan) because of the lack of a complete FDA scientific analysis of the drug and of peer-reviewed publication.

The survey highlights a growing willingness among employees to pay for premium lens options and a focus on early diagnosis of eye conditions, aligning with the broader trend of holistic well-being.
Regulators indicated that an additional trial would be needed to assess the efficacy of reproxalap to treat patients with dry eye. Aldeyra has submitted to the FDA a Special Protocol Assessment for the new trial.

By identifying patients with glaucoma who are socially vulnerable, clinicians might intervene sooner and prevent the worsening of visual impairment, he said.

Beovu led to greater improvement in retinal thickness and reduction of retinal fluid in patients with diabetic macular edema, a secondary endpoint in this head-to-head trial.

The announcement has triggered a wave of concerns among users of eye-care products, particularly in those designed to alleviate dry and irritated eyes.
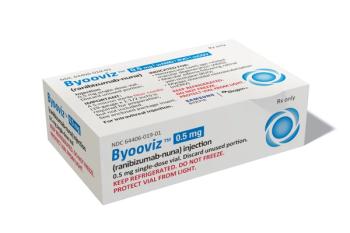
Byooviz was approved as the first biosimilar to Lucentis and launched with a list price of $1,130 per single-use vial.
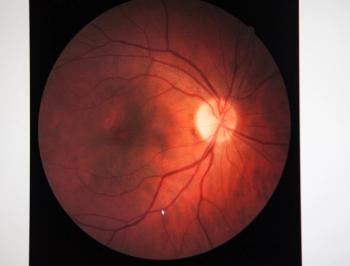
The FDA is currently reviewing a supplemental biologics license application for Vabysmo in patients with macular edema due to retinal vein occlusion. A decision from the FDA is expected in late 2023.
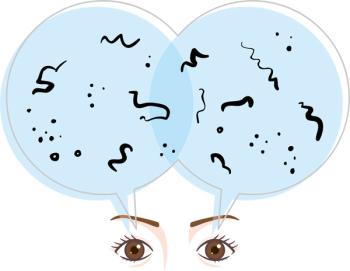
The authors of a case series reported in today's edition of JAMA Ophthalmology say silicone oil droplets from silicone used to lubricate the McKesson syringes is the most likely cause of the droplets presumed to cause the floaters. Of the 55 patients treated with Syfovre, 16 developed floaters.
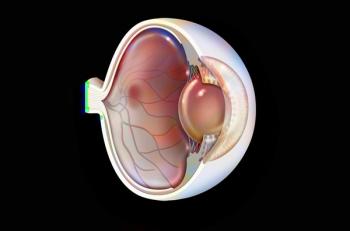
Animal studies of a new compound, which can be delivered by eye drop, have found that it can reverse epigenetic changes that lead to wet age-related macular degeneration.

Ryzumi is expected to be available in the first half of 2024. Pricing will be available around the time of launch.

Ryzumi is expected to be available in the first half of 2024. Pricing will be available around the time of launch.

Iyuzeh can be stored at room temperature. It has a list price of $299 for a month’s supply.

Iyuzeh is a preservative-free latanoprost product that can be stored at room temperature. It has a list price of $299 for a month’s supply.