
If approved, ADX-2191 could be the first marketed drug specifically for patients with primary vitreoretinal lymphoma. The FDA assigned a Prescription Drug User Fee Act date of June 21, 2023.

If approved, ADX-2191 could be the first marketed drug specifically for patients with primary vitreoretinal lymphoma. The FDA assigned a Prescription Drug User Fee Act date of June 21, 2023.
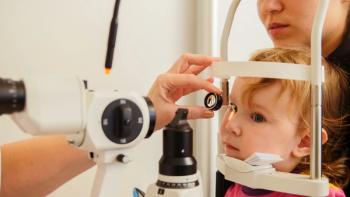
Disparities in access to pediatric eye care have increased over the past 15 years and are associated with lower socioeconomic status.

CSF-1 is a low-dose pilocarpine to treat patients with age-related blurry vision. The FDA has assigned a PDUFA goal date of Oct. 22, 2023

Starting with Avastin to treat patients with diabetic macular edema — and switching to Eylea if patients don’t have a response — could result in savings of more than $125 million.

Apellis’ Syfovre will have a list price of $2,190 per vial, and Medicare is expected to be the dominant payer.

Sedentary behavior has been linked with low-grade systemic inflammation, which could disrupt the ocular surface.
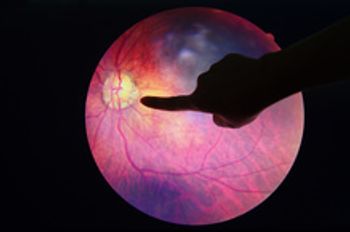
Avacincaptad pegol has the potential to slow the progression of geographic atrophy in age-related macular degeneration. The PDUFA goal date is Aug. 19, 2023.
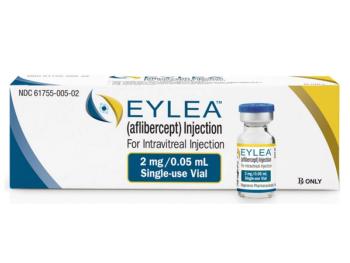
Eylea now also treats retinopathy of prematurity, a leading cause of childhood blindness worldwide.

If approved, reproxalap would be the first inhibitor of RASP to treat patients with dry eye disease. The FDA PDUFA date is Nov. 23, 2023.
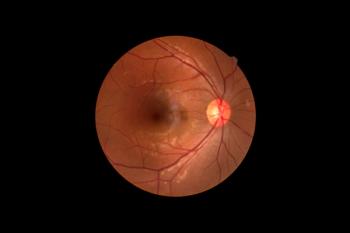
Scientists have created a lipid nanoparticle that was able to deliver mRNA to the retina, providing an opportunity for the development of additional gene therapies for the eye.

Researchers found that visual impairment is more prevalent among those who are older, Hispanic, non-White, and less educated and have lower incomes.
Girls were more likely to develop dry eye after surgery, and nondominant eyes are more likely to develop postoperative dry eye.

If approved, CSF-1 would be the second therapy to treat patients with presbyopia, or age-related blurry vision.

Iyuzeh is a preservative-free formulation of latanoprost and doesn’t need to be stored at refrigerated temperatures. It will be available in the second half of 2023.

The early response to neovascular age-related macular degeneration (nvAMD) treatment with aflibercept (Eyelea) was much greater than the response to bevacizumab (Avastin), in hopes of weaning those affected off the therapies sooner.

The APOE gene is known for its association in Alzheimer’s disease. Investigators are beginning to understand its role in age-related macular degeneration and other eye diseases.

In an umbrella review — a review of reviews — investigators found that so far, studies of ocular biomarkers to diagnose Alzheimer’s disease had important limitations. Longitudinal studies that use artificial intelligence could perhaps identify ocular biomarkers, the researchers suggest.
If approved, reproxalap would be the first inhibitor of RASP, which contributes to ocular inflammation and changes in tear lipid composition.

Appellis had submitted updated data for the pegcetacoplan in age-related macular degeneration, which is considered a major amendment to the NDA. The new PDUFA date is Feb. 26, 2023.
NIH researchers have created a 3D map of DNA within human retina cells and identified new gene variants that could be risk factors for age-related macular degeneration and glaucoma.

A bioengineered corneal implant made with collagen from pig’s skin could be an alternative to human transplants for those with keratoconus, a rare condition that can lead to blurry vision and blindness.

For patients with dry eyes, a cross-study comparison finds Tyrvaya may produce more natural tears than Xiidra.

Novaliq’s CyclASol uses the EyeSol technology that allows for improved bioavailability and better efficacy. The PDUFA target action date set by the FDA is June 8, 2023.

The FDA has assigned a target action date of Feb. 11, 2023.

This is the first study to use an artificial intelligence algorithm to break down visual field loss in new-onset glaucoma cases among U.S.-based population groups.