
AllianceRx Walgreens Prime study has found that patients taking Ocaliva may not be adherent to their treatment regimen for rare liver disease.

AllianceRx Walgreens Prime study has found that patients taking Ocaliva may not be adherent to their treatment regimen for rare liver disease.

A disconnect exists between payers and patients with rare diseases about including patient input in the formulary process for orphan drugs.

Prior authorization and step therapy can impact compliance and outcomes, according to a survey by the Pharmaceutical Research and Manufacturers of America.

Xipere is a targeted treatment that is delivered via an injection to the back of the eye to treat macular edema associated with uveitis, a form of eye inflammation.
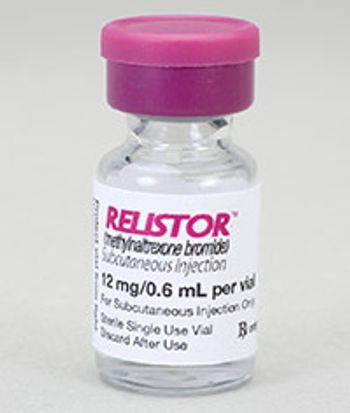
This real-world study found that patients who received Relistor SC during an emergency department visit for opioid induced constipation had lower costs and hospital lengths of stay.

Some generics have as much of a 10,000-times increase from the manufacturers weighted average manufacturers price to what a patient could pay at the pharmacy if they chose to pay the cash price.

The new code will be effective April 1, 2022.

Cystaran is a topical ophthalmic therapy that is used to treat a rare genetic disorder.

Del Doherty, co-founder of Prodigy Rx, discusses how the PBM aims to give payers control so they can lower costs and can improve clinical and return-to-work outcomes.

ICER analyzed two therapies — Cosela and plinabulin — to prevent chemotherapy-induced neutropenia and other myelosuppressive effects. Both moderately increased quality-adjusted life-years.

Allan Coukell, Civica’s senior vice president of public policy, discusses how the generic manufacturer is disrupting the market for insulins.

Evio Pharmacy Solutions plans to provide biosimilar alternatives for autoimmune and cancer therapeutics.
This expands commercial coverage to 118 million lives and Medicare coverage to 7.1 million lives.
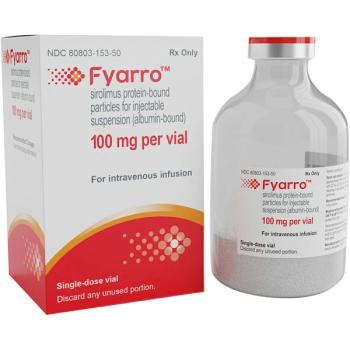
Aadi Bioscience recently launched Fyarro, the first FDA-approved therapy to treat an ultra-rare sarcoma.

Generics are now available for the three therapies: Cystadane, Selzentry, and AmBisome.
Once approved, the insulins will be available to patients for no more than $30 per vial.
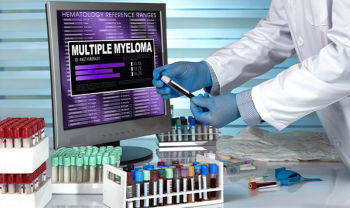
FDA approved Legend Biotech’s chimeric antigen receptor T-cell (CAR-T) treatment, Carvykti (ciltacabtagene autoleucel; cilta- cel), to treat relapsed or refractory multiple myeloma (RRMM) who have received four or more prior lines of therapy.
Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
The solution, available immediately to all large health plans, is meant to simplify care for patients with complex conditions.
The Biosimilars Forum Board of Directors recently appointed Juliana M. Reed as its new executive director and Formulary Watch got the scoop from Reed first hand.
Price increases on promising non–small cell lung cancer drugs despite evidence price competition raise concerns about affordability.

The new Medi-Cal Rx program that debuted Jan. 1 has alarmed health clinics that say the state's Medicaid will lose money and have to cut services.
United States healthcare spending rose 9.7% in 2020, while medication spending is increasing by more than 18%.

In a research letter published in JAMA Health Forum, researchers say most of the cost figures for Aduhelm have left out the additional MRI scans and other services that will be required because the drug is associated with the development of amyloid-related imaging abnormalities (ARIAs).

Lecanemab and donanemab, monoclonal antibodies for Alzheimer’s, are on the list of drugs in late-stage development that Clarivate analysts will have annual sales of a $1 billion or more in the next five years.